Sex- and Age-Specific Impact of ERK Loss Within the Pituitary Gonadotrope in Mice
- PMID: 29300908
- PMCID: PMC5802804
- DOI: 10.1210/en.2017-00653
Sex- and Age-Specific Impact of ERK Loss Within the Pituitary Gonadotrope in Mice
Erratum in
-
CORRIGENDUM FOR "Sex- and Age-Specific Impact of ERK Loss Within the Pituitary Gonadotrope in Mice".Endocrinology. 2018 May 1;159(5):1971. doi: 10.1210/en.2018-00291. Endocrinology. 2018. PMID: 29617748 Free PMC article. No abstract available.
Abstract
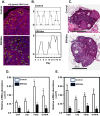
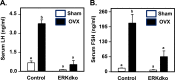
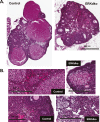
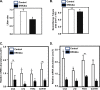
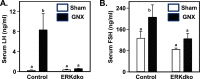
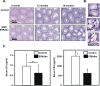
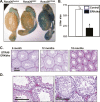
Extracellular signal-regulated kinase (ERK) signaling regulates hormone action in the reproductive axis, but specific mechanisms have yet to be completely elucidated. In the current study, ERK1 null and ERK2 floxed mice were combined with a gonadotropin-releasing hormone receptor (GnRHR)-internal ribosomal entry site-Cre (GRIC) driver. Female ERK double-knockout (ERKdko) animals were hypogonadotropic, resulting in anovulation and complete infertility. Transcript levels of four gonadotrope-specific genes (GnRHR and the three gonadotropin subunits) were reduced in pituitaries at estrus in ERKdko females, and the postcastration response to endogenous GnRH hyperstimulation was blunted. As females aged, they exhibited abnormal ovarian histology, as well as increased body weight. ERKdko males were initially less affected, showing moderate subfertility, up to 6 months of age. Male ERKdko mice also displayed a blunted response to endogenous GnRH following castration. By 12 months of age, ERKdko males had reduced testicular weights and sperm production. By 18 months of age, the ERKdko males displayed reduced testis and seminal vesicle weights, marked seminiferous tubule degeneration, and a 77% reduction in sperm production relative to controls. As the GRIC is also active in the male germ line, we examined the specific role of ERK loss in the testes using the stimulated by retinoic acid 8 (Stra8)-Cre driver. Whereas ERK loss in GRIC and Stra8 males resulted in comparable losses in sperm production, seminiferous tubule histological degeneration was only observed in the GRIC-ERKdko animals. Our data suggest that loss of ERK signaling and hypogonadotropism within the reproductive axis impacts fertility and gonadal aging.
Copyright © 2018 Endocrine Society.
Figures
Similar articles
-
Deletion of Gαq/11 or Gαs Proteins in Gonadotropes Differentially Affects Gonadotropin Production and Secretion in Mice.Endocrinology. 2022 Feb 1;163(2):bqab247. doi: 10.1210/endocr/bqab247. Endocrinology. 2022. PMID: 34864945 Free PMC article.
-
Conditional loss of ERK1 and ERK2 results in abnormal placentation and delayed parturition in the mouse.Sci Rep. 2019 Jul 3;9(1):9641. doi: 10.1038/s41598-019-45997-0. Sci Rep. 2019. PMID: 31270345 Free PMC article.
-
GnRH Receptor Expression and Reproductive Function Depend on JUN in GnRH Receptor‒Expressing Cells.Endocrinology. 2018 Mar 1;159(3):1496-1510. doi: 10.1210/en.2017-00844. Endocrinology. 2018. PMID: 29409045 Free PMC article.
-
GnRH signaling, the gonadotrope and endocrine control of fertility.Front Neuroendocrinol. 2010 Jul;31(3):322-40. doi: 10.1016/j.yfrne.2010.04.002. Epub 2010 May 6. Front Neuroendocrinol. 2010. PMID: 20451543 Free PMC article. Review.
-
Signaling by G-protein-coupled receptor (GPCR): studies on the GnRH receptor.Front Neuroendocrinol. 2009 Jan;30(1):10-29. doi: 10.1016/j.yfrne.2008.07.001. Epub 2008 Aug 5. Front Neuroendocrinol. 2009. PMID: 18708085 Review.
Cited by
-
GnRH-A Key Regulator of FSH.Endocrinology. 2019 Jan 1;160(1):57-67. doi: 10.1210/en.2018-00889. Endocrinology. 2019. PMID: 30517625 Free PMC article. Review.
-
ERK1/2-RSK2 Signaling in Regulation of ERα-Mediated Responses.Endocrinology. 2022 Sep 1;163(9):bqac106. doi: 10.1210/endocr/bqac106. Endocrinology. 2022. PMID: 35880639 Free PMC article. Review.
-
[Gandou Bushen Decoction improves spermatogenesis and promotes spermatogenic cell proliferation in Wilson disease TX mice by activating testicular ERK signaling pathway].Nan Fang Yi Ke Da Xue Xue Bao. 2024 Nov 20;44(11):2063-2073. doi: 10.12122/j.issn.1673-4254.2024.11.02. Nan Fang Yi Ke Da Xue Xue Bao. 2024. PMID: 39623261 Free PMC article. Chinese.
-
Deletion of Gαq/11 or Gαs Proteins in Gonadotropes Differentially Affects Gonadotropin Production and Secretion in Mice.Endocrinology. 2022 Feb 1;163(2):bqab247. doi: 10.1210/endocr/bqab247. Endocrinology. 2022. PMID: 34864945 Free PMC article.
-
Gonadotrope-specific deletion of the BMP type 2 receptor does not affect reproductive physiology in mice†‡.Biol Reprod. 2020 Mar 13;102(3):639-646. doi: 10.1093/biolre/ioz206. Biol Reprod. 2020. PMID: 31724029 Free PMC article.
References
-
- Brown JL, Roberson M. Novel insights into gonadotropin-releasing hormone action in the pituitary gonadotrope. Semin Reprod Med. 2017;35(2):130–138. - PubMed
-
- Clarke IJ, Cummins JT. The temporal relationship between gonadotropin releasing hormone (GnRH) and luteinizing hormone (LH) secretion in ovariectomized ewes. Endocrinology. 1982;111(5):1737–1739. - PubMed
-
- Knobil E. The GnRH pulse generator. Am J Obstet Gynecol. 1990;163(5):1721–1727. - PubMed
-
- Wen S, Schwarz JR, Niculescu D, Dinu C, Bauer CK, Hirdes W, Boehm U. Functional characterization of genetically labeled gonadotropes. Endocrinology. 2008;149(6):2701–2711. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

