Marine Alkylpurines: A Promising Group of Bioactive Marine Natural Products
- PMID: 29301246
- PMCID: PMC5793054
- DOI: 10.3390/md16010006
Marine Alkylpurines: A Promising Group of Bioactive Marine Natural Products
Abstract
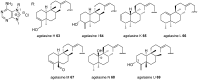
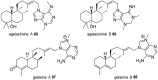
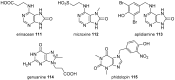
Marine secondary metabolites with a purine motif in their structure are presented in this review. The alkylpurines are grouped according to the size of the alkyl substituents and their location on the purine ring. Aspects related to the marine source, chemical structure and biological properties are considered together with synthetic approaches towards the natural products and bioactive analogues. This review contributes to studies of structure-activity relationships for these metabolites and highlights the potential of the sea as a source of new lead compounds in diverse therapeutic fields.
Keywords: alkylpurines; antimicrobial activity; cytotoxicity; marine natural products; methylpurines; purines; terpenylpurines.
Conflict of interest statement
The authors declare no conflict of interest. The founding sponsors had no role in the design of the study, in the data collection, and in the writing and decision to publish the manuscript.
Figures










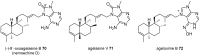























Similar articles
-
Natural Products Repertoire of the Red Sea.Mar Drugs. 2020 Sep 4;18(9):457. doi: 10.3390/md18090457. Mar Drugs. 2020. PMID: 32899763 Free PMC article. Review.
-
Chemical Diversity and Biological Properties of Secondary Metabolites from Sea Hares of Aplysia Genus.Mar Drugs. 2016 Feb 19;14(2):39. doi: 10.3390/md14020039. Mar Drugs. 2016. PMID: 26907303 Free PMC article. Review.
-
Bioactive Natural Products from the Red Sea.Mar Drugs. 2021 May 21;19(6):289. doi: 10.3390/md19060289. Mar Drugs. 2021. PMID: 34064008 Free PMC article.
-
Antimycobacterial Metabolites from Marine Invertebrates.Arch Pharm (Weinheim). 2016 Oct;349(10):763-773. doi: 10.1002/ardp.201600128. Epub 2016 Aug 26. Arch Pharm (Weinheim). 2016. PMID: 27561360 Review.
-
Marine Bacteria: A Source of Novel Bioactive Natural Products.Curr Med Chem. 2024;31(41):6842-6854. doi: 10.2174/0929867331666230821102521. Curr Med Chem. 2024. PMID: 37605398 Review.
Cited by
-
Bioactive Metabolites of Marine Origin Have Unusual Effects on Model Membrane Systems.Mar Drugs. 2020 Feb 19;18(2):125. doi: 10.3390/md18020125. Mar Drugs. 2020. PMID: 32092956 Free PMC article.
-
Geobarrettin D, a Rare Herbipoline-Containing 6-Bromoindole Alkaloid from Geodia barretti.Molecules. 2023 Mar 24;28(7):2937. doi: 10.3390/molecules28072937. Molecules. 2023. PMID: 37049700 Free PMC article.
-
Antibacterial Natural Halimanes: Potential Source of Novel Antibiofilm Agents.Molecules. 2020 Apr 8;25(7):1707. doi: 10.3390/molecules25071707. Molecules. 2020. PMID: 32276434 Free PMC article. Review.
-
Function of Adenosine 2A Receptor in High-Fat Diet-Induced Peripheral Neuropathy.J Diabetes Res. 2020 May 22;2020:7856503. doi: 10.1155/2020/7856503. eCollection 2020. J Diabetes Res. 2020. PMID: 32566683 Free PMC article.
-
Historical and Current Adenosine Receptor Agonists in Preclinical and Clinical Development.Front Cell Neurosci. 2019 Mar 28;13:124. doi: 10.3389/fncel.2019.00124. eCollection 2019. Front Cell Neurosci. 2019. PMID: 30983976 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

