Skipping Multiple Exons to Treat DMD-Promises and Challenges
- PMID: 29301272
- PMCID: PMC5874658
- DOI: 10.3390/biomedicines6010001
Skipping Multiple Exons to Treat DMD-Promises and Challenges
Abstract
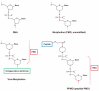
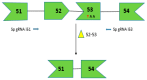
Duchenne muscular dystrophy (DMD) is a lethal disorder caused by mutations in the DMD gene. Antisense-mediated exon-skipping is a promising therapeutic strategy that makes use of synthetic nucleic acids to skip frame-disrupting exon(s) and allows for short but functional protein expression by restoring the reading frame. In 2016, the U.S. Food and Drug Administration (FDA) approved eteplirsen, which skips DMD exon 51 and is applicable to approximately 13% of DMD patients. Multiple exon skipping, which is theoretically applicable to 80-90% of DMD patients in total, have been demonstrated in animal models, including dystrophic mice and dogs, using cocktail antisense oligonucleotides (AOs). Although promising, current drug approval systems pose challenges for the use of a cocktail AO. For example, both exons 6 and 8 need to be skipped to restore the reading frame in dystrophic dogs. Therefore, the cocktail of AOs targeting these exons has a combined therapeutic effect and each AO does not have a therapeutic effect by itself. The current drug approval system is not designed to evaluate such circumstances, which are completely different from cocktail drug approaches in other fields. Significant changes are needed in the drug approval process to promote the cocktail AO approach.
Keywords: Clustered Regularly Interspaced Short Palindromic Repeat/CRISPR associated protein 9 (CRISPR/Cas9)-mediated genome editing; Duchenne/Becker muscular dystrophy (DMD/BMD); actin binding domain (ABD); antisense oligonucleotides (AOs); canine X-linked muscular dystrophy (CXMD); eteplirsen; golden retriever muscular dystrophy (GRMD); golodirsen; morpholino); multi-exon skipping; phosphorodiamidate morpholino oligomer (PMO.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Koenig M., Hoffman E.P., Bertelson C.J., Monaco A.P., Feener C., Kunkel L.M. Complete cloning of the Duchenne muscular dystrophy (DMD) cDNA and preliminary genomic organization of the DMD gene in normal and affected individuals. Cell. 1987;50:509–517. doi: 10.1016/0092-8674(87)90504-6. - DOI - PubMed
-
- Matsumura K., Ohlendieck K., Ionasescu V.V., Tome F.M., Nonaka I., Burghes A.H., Mora M., Kaplan J.C., Fardeau M., Campbell K.P. The role of the dystrophin-glycoprotein complex in the molecular pathogenesis of muscular dystrophies. Neuromuscul. Disord. NMD. 1993;3:533–535. doi: 10.1016/0960-8966(93)90110-6. - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

