The Sodium-Glucose Cotransporter 2 Inhibitor Dapagliflozin Prevents Renal and Liver Disease in Western Diet Induced Obesity Mice
- PMID: 29301371
- PMCID: PMC5796086
- DOI: 10.3390/ijms19010137
The Sodium-Glucose Cotransporter 2 Inhibitor Dapagliflozin Prevents Renal and Liver Disease in Western Diet Induced Obesity Mice
Abstract
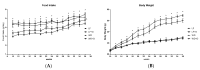
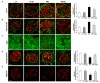
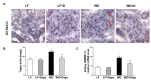
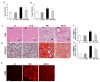
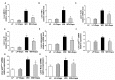
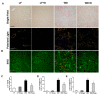
Obesity and obesity related kidney and liver disease have become more prevalent over the past few decades, especially in the western world. Sodium-glucose cotransporter 2 (SGLT2) inhibitors are a new class of antidiabetic agents with promising effects on cardiovascular and renal function. Given SGLT2 inhibitors exert both anti-diabetic and anti-obesity effects by promoting urinary excretion of glucose and subsequent caloric loss, we investigated the effect of the highly selective renal SGLT2 inhibitor dapagliflozin in mice with Western diet (WD) induced obesity. Low fat (LF) diet or WD-fed male C57BL/6J mice were treated with dapagliflozin for 26 weeks. Dapagliflozin attenuated the WD-mediated increases in body weight, plasma glucose and plasma triglycerides. Treatment with dapagliflozin prevented podocyte injury, glomerular pathology and renal fibrosis determined by second harmonic generation (SHG), nephrin, synaptopodin, collagen IV, and fibronectin immunofluorescence microscopy. Oil Red O staining showed dapagliflozin also decreased renal lipid accumulation associated with decreased SREBP-1c mRNA abundance. Moreover, renal inflammation and oxidative stress were lower in the dapagliflozin-treated WD-fed mice than in the untreated WD-fed mice. In addition, dapagliflozin decreased serum alanine aminotransferase (ALT) and aspartate aminotransferase (AST), hepatic lipid accumulation as determined by H&E and Oil Red O staining, and Coherent Anti-Stokes Raman Scattering (CARS) microscopy, and hepatic fibrosis as determined by picrosirius red (PSR) staining and TPE-SHG microscopy in WD-fed mice. Thus, our study demonstrated that the co-administration of the SGLT2 inhibitor dapagliflozin attenuates renal and liver disease during WD feeding of mice.
Keywords: SGLT2; dapagliflozin; fibrosis; inflammation; lipid; obesity.
Conflict of interest statement
This study was supported by an Investigator Initiated Study (IIS) grant support from Merck to the University of Colorado and Moshe Levi. No other conflicts of interest are declared by the author(s).
Figures


References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

