Development and content validation of a patient-reported endometriosis pain daily diary
- PMID: 29301557
- PMCID: PMC5753477
- DOI: 10.1186/s12955-017-0819-1
Development and content validation of a patient-reported endometriosis pain daily diary
Abstract
Background: Endometriosis is a common gynecological disorder that causes inflammation and pelvic pain. Endometriosis-related pain is best captured with patient-reported outcome (PRO) measures, however, assessment of endometriosis-related pain in clinical trials has been difficult in the absence of a reliable and valid PRO instrument. We describe the development of the Endometriosis Pain Daily Diary (EPDD), an electronic PRO developed as a survey instrument to assess endometriosis-related pain and its impact on patients' lives.
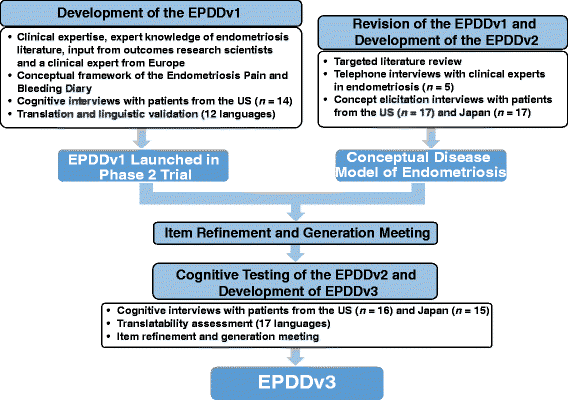
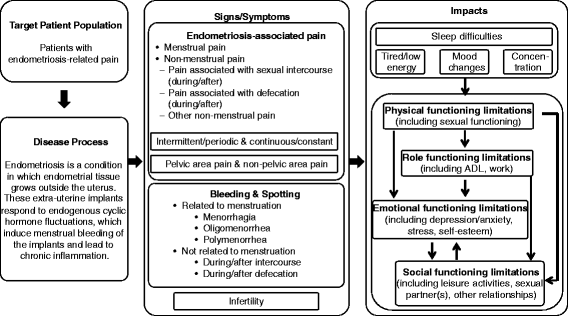
Methods: The EPDD was initially developed on the basis of an existing Endometriosis Pain and Bleeding Diary, a targeted review of relevant literature, clinical expert interviews, and open-ended (concept elicitation) patient interviews in the United States (US) and Japan which captured patients' experience with endometriosis. Cognitive interviews of patients with endometriosis were conducted to evaluate patient comprehension of the EPDD items. A conceptual model of endometriosis was developed, and meetings with US and European regulatory authorities provided feedback for validating the EPDD in the context of clinical trials. Translatability assessments of the EPDD were conducted to confirm its appropriate interpretation and ease of completion across 17 languages.
Results: The iterative development progressed through three versions of the instrument. The EPDDv1 included 18 items relating to dysmenorrhea/pelvic pain, dyspareunia and sexual activity, bleeding, hot flashes, daily activities, and use of rescue medication. The EPDDv2 was a larger 43-item survey tested in cognitive interviews and subsequently revised to yield the current 11-item EPDDv3, consisting of five core items relating to dysmenorrhea, non-menstrual pelvic pain, and dyspareunia, and six extension items relating to sexual activity, daily activities, and use of rescue medication.
Conclusions: The EPDD is a PRO for the evaluation of endometriosis-related pain and its associated impacts on patients' lives. The EPDD represents an important step in providing a PRO that is relevant to patients with endometriosis-related pain in the context of a clinical study setting (ie, fit-for-purpose), designed to evaluate pain associated with endometriosis, including regulatory agency support for its further exploration in clinical trials.
Keywords: Dysmenorrhea; Endometriosis; Patient-reported outcome measures; Pelvic pain.
Conflict of interest statement
Ethics approval and consent to participate
Institutional review board approval for the study protocol, the informed consent form, and data collection forms for interviews with patients in the US was provided by Quorum Review (Seattle, WA).
Consent for publication
Not applicable.
Competing interests
Floortje E. van Nooten was employed by Astellas at the time of work. Jen Cline, Celeste A. Elash, Jean Paty, and Matthew Reaney were employed by ERT at the time of this work; Jean Paty was also employed by Quintiles.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures
Similar articles
-
Development and Validation of the Endometriosis Daily Pain Impact Diary Items to Assess Dysmenorrhea and Nonmenstrual Pelvic Pain.Reprod Sci. 2018 Nov;25(11):1567-1576. doi: 10.1177/1933719118789509. Epub 2018 Jul 22. Reprod Sci. 2018. PMID: 30033855
-
Patients' and clinicians' perspectives on item importance, scoring, and clinically meaningful differences for the Endometriosis Symptom Diary (ESD) and Endometriosis Impact Scale (EIS).Health Qual Life Outcomes. 2021 Jan 6;19(1):7. doi: 10.1186/s12955-020-01579-7. Health Qual Life Outcomes. 2021. PMID: 33407560 Free PMC article.
-
The development and validation of the daily electronic Endometriosis Pain and Bleeding Diary.Health Qual Life Outcomes. 2010 Jul 2;8:64. doi: 10.1186/1477-7525-8-64. Health Qual Life Outcomes. 2010. PMID: 20598144 Free PMC article.
-
The CDI-DaySyms: Content Development of a New Patient-Reported Outcome Questionnaire for Symptoms of Clostridium difficile Infection.Value Health. 2018 Apr;21(4):441-448. doi: 10.1016/j.jval.2017.08.3017. Epub 2017 Nov 7. Value Health. 2018. PMID: 29680101 Review.
-
[Management of endometriosis: clinical and biological assessment].J Gynecol Obstet Biol Reprod (Paris). 2007 Apr;36(2):119-28. doi: 10.1016/j.jgyn.2006.12.020. Epub 2007 Feb 2. J Gynecol Obstet Biol Reprod (Paris). 2007. PMID: 17276015 Review. French.
Cited by
-
Development and content validation of two new patient-reported outcome measures for endometriosis: the Endometriosis Symptom Diary (ESD) and Endometriosis Impact Scale (EIS).J Patient Rep Outcomes. 2020 Feb 18;4(1):13. doi: 10.1186/s41687-020-0177-3. J Patient Rep Outcomes. 2020. PMID: 32072316 Free PMC article.
-
Understanding data visualization techniques in qualitative studies used to develop and validate patient-reported outcome measures: a targeted literature review.Qual Life Res. 2025 Jul;34(7):2031-2048. doi: 10.1007/s11136-025-03964-5. Epub 2025 Apr 25. Qual Life Res. 2025. PMID: 40279025 Review.
-
The effectiveness of a modified Gui Zhi Fu Ling Wan formulation (Gynoclear™) for the treatment of endometriosis: a study protocol for a placebo-controlled, double-blind, randomised controlled trial.Trials. 2021 Apr 21;22(1):299. doi: 10.1186/s13063-021-05265-x. Trials. 2021. PMID: 33883001 Free PMC article.
-
The ENDOPAIN 4D Questionnaire: A New Validated Tool for Assessing Pain in Endometriosis.J Clin Med. 2021 Jul 21;10(15):3216. doi: 10.3390/jcm10153216. J Clin Med. 2021. PMID: 34362000 Free PMC article.
-
Endometriosis Symptoms and Their Impacts on the Daily Lives of US Women: Results from an Interview Study.Int J Womens Health. 2023 Jun 1;15:893-904. doi: 10.2147/IJWH.S409733. eCollection 2023. Int J Womens Health. 2023. PMID: 37283994 Free PMC article.
References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

