Functional and clinical relevance of VLA-4 (CD49d/CD29) in ibrutinib-treated chronic lymphocytic leukemia
- PMID: 29301866
- PMCID: PMC5789417
- DOI: 10.1084/jem.20171288
Functional and clinical relevance of VLA-4 (CD49d/CD29) in ibrutinib-treated chronic lymphocytic leukemia
Abstract
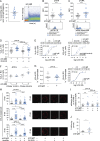
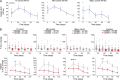
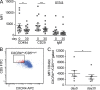
The Bruton's tyrosine kinase (BTK) inhibitor ibrutinib, which antagonizes B cell receptor (BCR) signals, demonstrates remarkable clinical activity in chronic lymphocytic leukemia (CLL). The lymphocytosis experienced by most patients under ibrutinib has previously been attributed to inhibition of BTK-dependent integrin and chemokine cues operating to retain the tumor cells in nodal compartments. Here, we show that the VLA-4 integrin, as expressed by CD49d-positive CLL, can be inside-out activated upon BCR triggering, thus reinforcing the adhesive capacities of CLL cells. In vitro and in vivo ibrutinib treatment, although reducing the constitutive VLA-4 activation and cell adhesion, can be overcome by exogenous BCR triggering in a BTK-independent manner involving PI3K. Clinically, in three independent ibrutinib-treated CLL cohorts, CD49d expression identifies cases with reduced lymphocytosis and inferior nodal response and behaves as independent predictor of shorter progression-free survival, suggesting the retention of CD49d-expressing CLL cells in tissue sites via activated VLA-4. Evaluation of CD49d expression should be incorporated in the characterization of CLL undergoing therapy with BCR inhibitors.
© 2018 Tissino et al.
Figures




References
-
- Arana E., Vehlow A., Harwood N.E., Vigorito E., Henderson R., Turner M., Tybulewicz V.L., and Batista F.D.. 2008b Activation of the small GTPase Rac2 via the B cell receptor regulates B cell adhesion and immunological-synapse formation. Immunity. 28:88–99. 10.1016/j.immuni.2007.12.003 - DOI - PubMed
-
- Brown J.R., Hillmen P., O’Brien S., Barrientos J.C., Reddy N.M., Coutre S.E., Tam C.S., Mulligan S.P., Jaeger U., Barr P.M., et al. . 2017. Extended follow-up and impact of high-risk prognostic factors from the phase 3 RESONATE study in patients with previously treated CLL/SLL. Leukemia. 10.1038/leu.2017.175 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

