Selection-Enhanced Mutagenesis of lac Genes Is Due to Their Coamplification with dinB Encoding an Error-Prone DNA Polymerase
- PMID: 29301907
- PMCID: PMC5844319
- DOI: 10.1534/genetics.117.300409
Selection-Enhanced Mutagenesis of lac Genes Is Due to Their Coamplification with dinB Encoding an Error-Prone DNA Polymerase
Abstract
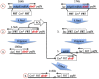
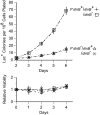
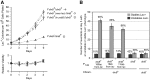
To test whether growth limitation induces mutations, Cairns and Foster constructed an Escherichia coli strain whose mutant lac allele provides 1-2% of normal ability to use lactose. This strain cannot grow on lactose, but produces ∼50 Lac+ revertant colonies per 108 plated cells over 5 days. About 80% of revertants carry a stable lac+ mutation made by the error-prone DinB polymerase, which may be induced during growth limitation; 10% of Lac+ revertants are stable but form without DinB; and the remaining 10% grow by amplifying their mutant lac allele and are unstably Lac+ Induced DinB mutagenesis has been explained in two ways: (1) upregulation of dinB expression in nongrowing cells ("stress-induced mutagenesis") or (2) selected local overreplication of the lac and dinB+ genes on lactose medium (selected amplification) in cells that are not dividing. Transcription of dinB is necessary but not sufficient for mutagenesis. Evidence is presented that DinB enhances reversion only when encoded somewhere on the F'lac plasmid that carries the mutant lac gene. A new model will propose that rare preexisting cells (1 in a 1000) have ∼10 copies of the F'lac plasmid, providing them with enough energy to divide, mate, and overreplicate their F'lac plasmid under selective conditions. In these clones, repeated replication of F'lac in nondividing cells directs opportunities for lac reversion and increases the copy number of the dinB+ gene. Amplification of dinB+ increases the error rate of replication and increases the number of lac+ revertants. Thus, reversion is enhanced in nondividing cells not by stress-induced mutagenesis, but by selected coamplification of the dinB and lac genes, both of which happen to lie on the F'lac plasmid.
Keywords: Escherichia coli; adaptive mutation; copy number variant; dinB; error-prone polymerase; gene amplification; lactose operon; local overreplication; mutagenesis; plasmid.
Copyright © 2018 by the Genetics Society of America.
Figures



Similar articles
-
Selection and Plasmid Transfer Underlie Adaptive Mutation in Escherichia coli.Genetics. 2018 Nov;210(3):821-841. doi: 10.1534/genetics.118.301347. Epub 2018 Sep 7. Genetics. 2018. PMID: 30194073 Free PMC article.
-
Selective Inbreeding: Genetic Crosses Drive Apparent Adaptive Mutation in the Cairns-Foster System of Escherichia coli.Genetics. 2020 Feb;214(2):333-354. doi: 10.1534/genetics.119.302754. Epub 2019 Dec 6. Genetics. 2020. PMID: 31810989 Free PMC article.
-
The Origin of Mutants Under Selection: How Natural Selection Mimics Mutagenesis (Adaptive Mutation).Cold Spring Harb Perspect Biol. 2015 Jul 1;7(7):a018176. doi: 10.1101/cshperspect.a018176. Cold Spring Harb Perspect Biol. 2015. PMID: 26134316 Free PMC article. Review.
-
Plasmid copy number underlies adaptive mutability in bacteria.Genetics. 2014 Nov;198(3):919-33. doi: 10.1534/genetics.114.170068. Epub 2014 Aug 29. Genetics. 2014. PMID: 25173846 Free PMC article.
-
Adaptive mutation and amplification in Escherichia coli: two pathways of genome adaptation under stress.Res Microbiol. 2004 Jun;155(5):352-9. doi: 10.1016/j.resmic.2004.01.020. Res Microbiol. 2004. PMID: 15207867 Review.
Cited by
-
Selection and Plasmid Transfer Underlie Adaptive Mutation in Escherichia coli.Genetics. 2018 Nov;210(3):821-841. doi: 10.1534/genetics.118.301347. Epub 2018 Sep 7. Genetics. 2018. PMID: 30194073 Free PMC article.
-
Transposable element insertions in fission yeast drive adaptation to environmental stress.Genome Res. 2019 Jan;29(1):85-95. doi: 10.1101/gr.239699.118. Epub 2018 Dec 12. Genome Res. 2019. PMID: 30541785 Free PMC article.
-
RETRACTED ARTICLE: Mutagenesis combined with fermentation optimization to enhance gibberellic acid GA3 yield in Fusarium fujikuroi.Bioresour Bioprocess. 2022 Oct 5;9(1):106. doi: 10.1186/s40643-022-00595-3. Bioresour Bioprocess. 2022. Retraction in: Bioresour Bioprocess. 2023 Apr 3;10(1):23. doi: 10.1186/s40643-023-00644-5. PMID: 38647889 Free PMC article. Retracted.
-
Selective Inbreeding: Genetic Crosses Drive Apparent Adaptive Mutation in the Cairns-Foster System of Escherichia coli.Genetics. 2020 Feb;214(2):333-354. doi: 10.1534/genetics.119.302754. Epub 2019 Dec 6. Genetics. 2020. PMID: 31810989 Free PMC article.
References
-
- Andersson D. I., Slechta E. S., Roth J. R., 1998. Evidence that gene amplification underlies adaptive mutability of the bacterial lac operon. Science 282: 1133–1135. - PubMed
-
- Bailone A., Bäckman A., Sommer S., Célérier J., Bagdasarian M. M., et al. , 1988. PsiB polypeptide prevents activation of RecA protein in Escherichia coli. Mol. Gen. Genet. 214: 389–395. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

