Injection of embryonic stem cell derived macrophages ameliorates fibrosis in a murine model of liver injury
- PMID: 29302350
- PMCID: PMC5677947
- DOI: 10.1038/s41536-017-0017-0
Injection of embryonic stem cell derived macrophages ameliorates fibrosis in a murine model of liver injury
Erratum in
-
Erratum: Author Correction: Injection of embryonic stem cell-derived macrophages ameliorates fibrosis in a murine model of liver injury.NPJ Regen Med. 2017 Nov 13;2:31. doi: 10.1038/s41536-017-0035-y. eCollection 2017. NPJ Regen Med. 2017. PMID: 29303159 Free PMC article.
Abstract
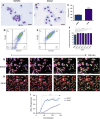
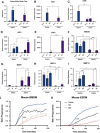
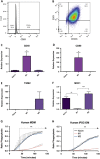
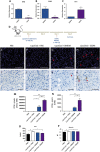
Chronic liver injury can be caused by viral hepatitis, alcohol, obesity, and metabolic disorders resulting in fibrosis, hepatic scarring, and cirrhosis. Novel therapies are urgently required and previous work has demonstrated that treatment with bone marrow derived macrophages can improve liver regeneration and reduce fibrosis in a murine model of hepatic injury and fibrosis. Here, we describe a protocol whereby pure populations of therapeutic macrophages can be produced in vitro from murine embryonic stem cells on a large scale. Embryonic stem cell derived macrophages display comparable morphology and cell surface markers to bone marrow derived macrophages but our novel imaging technique revealed that their phagocytic index was significantly lower. Differences were also observed in their response to classical induction protocols with embryonic stem cell derived macrophages having a reduced response to lipopolysaccharide and interferon gamma and an enhanced response to IL4 compared to bone marrow derived macrophages. When their therapeutic potential was assessed in a murine, carbon tetrachloride-induced injury and fibrosis model, embryonic stem cell derived macrophages significantly reduced the amount of hepatic fibrosis to 50% of controls, down-regulated the number of fibrogenic myofibroblasts and activated liver progenitor cells. To our knowledge, this is the first study that demonstrates a therapeutic effect of macrophages derived in vitro from pluripotent stem cells in a model of liver injury. We also found that embryonic stem cell derived macrophages repopulated the Kupffer cell compartment of clodronate-treated mice more efficiently than bone marrow derived macrophages, and expressed comparatively lower levels of Myb and Ccr2, indicating that their phenotype is more comparable to tissue-resident rather than monocyte-derived macrophages.
Conflict of interest statement
The authors declare that they have no competing financial interests.
Figures


Comment in
-
Macrophages come on tap for liver fibrosis therapy.Hepatology. 2018 Sep;68(3):1194-1196. doi: 10.1002/hep.29867. Epub 2018 May 14. Hepatology. 2018. PMID: 29500911 No abstract available.
References
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

