gPKPDSim: a SimBiology®-based GUI application for PKPD modeling in drug development
- PMID: 29302838
- PMCID: PMC5845055
- DOI: 10.1007/s10928-017-9562-9
gPKPDSim: a SimBiology®-based GUI application for PKPD modeling in drug development
Abstract
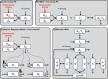
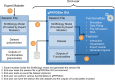
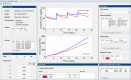
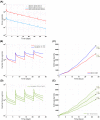
Modeling and simulation (M&S) is increasingly used in drug development to characterize pharmacokinetic-pharmacodynamic (PKPD) relationships and support various efforts such as target feasibility assessment, molecule selection, human PK projection, and preclinical and clinical dose and schedule determination. While model development typically require mathematical modeling expertise, model exploration and simulations could in many cases be performed by scientists in various disciplines to support the design, analysis and interpretation of experimental studies. To this end, we have developed a versatile graphical user interface (GUI) application to enable easy use of any model constructed in SimBiology® to execute various common PKPD analyses. The MATLAB®-based GUI application, called gPKPDSim, has a single screen interface and provides functionalities including simulation, data fitting (parameter estimation), population simulation (exploring the impact of parameter variability on the outputs of interest), and non-compartmental PK analysis. Further, gPKPDSim is a user-friendly tool with capabilities including interactive visualization, exporting of results and generation of presentation-ready figures. gPKPDSim was designed primarily for use in preclinical and translational drug development, although broader applications exist. gPKPDSim is a MATLAB®-based open-source application and is publicly available to download from MATLAB® Central™. We illustrate the use and features of gPKPDSim using multiple PKPD models to demonstrate the wide applications of this tool in pharmaceutical sciences. Overall, gPKPDSim provides an integrated, multi-purpose user-friendly GUI application to enable efficient use of PKPD models by scientists from various disciplines, regardless of their modeling expertise.
Keywords: Graphical-user-interface; Modeling and simulation; Non-compartmental analysis; Pharmacokinetics and pharmacodynamics models.
Figures
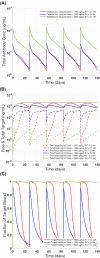
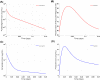
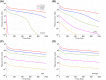
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

