Effects of Fluoxetine on Hippocampal Neurogenesis and Neuroprotection in the Model of Global Cerebral Ischemia in Rats
- PMID: 29304004
- PMCID: PMC5796111
- DOI: 10.3390/ijms19010162
Effects of Fluoxetine on Hippocampal Neurogenesis and Neuroprotection in the Model of Global Cerebral Ischemia in Rats
Abstract
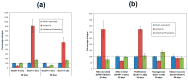
A selective serotonin reuptake inhibitor, fluoxetine, has recently attracted a significant interest as a neuroprotective therapeutic agent. There is substantial evidence of improved neurogenesis under fluoxetine treatment of brain ischemia in animal stroke models. We studied long-term effects of fluoxetine treatment on hippocampal neurogenesis, neuronal loss, inflammation, and functional recovery in a new model of global cerebral ischemia (GCI). Brain ischemia was induced in adult Wistar male rats by transient occlusion of three main vessels originating from the aortic arch and providing brain blood supply. Fluoxetine was injected intraperitoneally in a dose of 20 mg/kg for 10 days after surgery. To evaluate hippocampal neurogenesis at time points 10 and 30 days, 5-Bromo-2'-deoxyuridine was injected at days 8-10 after GCI. According to our results, 10-day fluoxetine injections decreased neuronal loss and inflammation, improved survival and functional recovery of animals, enhanced neurogenesis, and prevented an early pathological increase in neural stem cell recruitment in the subgranular zone (SGZ) of the hippocampus without reducing the number of mature neurons at day 30 after GCI. In summary, this study suggests that fluoxetine may provide a promising therapy in cerebral ischemia due to its neuroprotective, anti-inflammatory, and neurorestorative effect.
Keywords: CA1; antidepressants; dentate gyrus; fluoxetine; global brain ischemia; hippocampus; inflammation; neural stem cells; neurogenesis; rats.
Conflict of interest statement
The authors declare no conflict of interest.
Figures










References
-
- Possamai F., dos Santos J., Walber T., Marcon J.C., dos Santos T.S., Lino de Oliveira C. Influence of enrichment on behavioral and neurogenic effects of antidepressants in Wistar rats submitted to repeated forced swim test. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2015;58:15–21. doi: 10.1016/j.pnpbp.2014.10.017. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

