Tumor targeted delivery of doxorubicin in malignant peripheral nerve sheath tumors
- PMID: 29304038
- PMCID: PMC5755733
- DOI: 10.1371/journal.pone.0181529
Tumor targeted delivery of doxorubicin in malignant peripheral nerve sheath tumors
Abstract
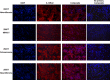
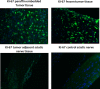
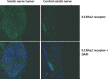
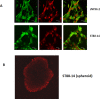
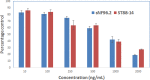
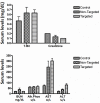
Peripheral nerve sheath tumors are benign tumors that have the potential to transform into malignant peripheral nerve sheath tumors (MPNSTs). Interleukin-13 receptor alpha 2 (IL13Rα2) is a cancer associated receptor expressed in glioblastoma and other invasive cancers. We analyzed IL13Rα2 expression in several MPNST cell lines including the STS26T cell line, as well as in several peripheral nerve sheath tumors to utilize the IL13Rα2 receptor as a target for therapy. In our studies, we demonstrated the selective expression of IL13Rα2 in several peripheral nerve sheath tumors by immunohistochemistry (IHC) and immunoblots. We established a sciatic nerve MPNST mouse model in NIH III nude mice using a luciferase transfected STS26T MPNST cell line. Similarly, analysis of the mouse sciatic nerves after tumor induction revealed significant expression of IL13Rα2 by IHC when compared to a normal sciatic nerve. IL13 conjugated liposomal doxorubicin was formulated and shown to bind and internalized in the MPNST cell culture model demonstrating cytotoxic effect. Our subsequent in vivo investigation in the STS26T MPNST sciatic nerve tumor model indicated that IL13 conjugated liposomal doxorubicin (IL13LIPDXR) was more effective in inhibiting tumor progression compared to unconjugated liposomal doxorubicin (LIPDXR). This further supports that IL13 receptor targeted nanoliposomes is a potential approach for treating MPNSTs.
Conflict of interest statement
Figures



Similar articles
-
Improved therapeutic activity of folate-targeted liposomal doxorubicin in folate receptor-expressing tumor models.Cancer Chemother Pharmacol. 2010 May;66(1):43-52. doi: 10.1007/s00280-009-1132-4. Epub 2009 Sep 25. Cancer Chemother Pharmacol. 2010. PMID: 19779718
-
A novel ligand delivery system to non-invasively visualize and therapeutically exploit the IL13Rα2 tumor-restricted biomarker.Neuro Oncol. 2012 Oct;14(10):1239-53. doi: 10.1093/neuonc/nos211. Epub 2012 Sep 5. Neuro Oncol. 2012. PMID: 22952195 Free PMC article.
-
Antitumor effects of 4-methylumbelliferone, a hyaluronan synthesis inhibitor, on malignant peripheral nerve sheath tumor.Int J Cancer. 2017 Jan 15;140(2):469-479. doi: 10.1002/ijc.30460. Epub 2016 Oct 18. Int J Cancer. 2017. PMID: 27706810
-
Non-cytotoxic systemic treatment in malignant peripheral nerve sheath tumors (MPNST): A systematic review from bench to bedside.Crit Rev Oncol Hematol. 2019 Jun;138:223-232. doi: 10.1016/j.critrevonc.2019.04.007. Epub 2019 Apr 19. Crit Rev Oncol Hematol. 2019. PMID: 31092379
-
Significance of interleukin-13 receptor alpha 2-targeted glioblastoma therapy.Neuro Oncol. 2014 Oct;16(10):1304-12. doi: 10.1093/neuonc/nou045. Epub 2014 Apr 10. Neuro Oncol. 2014. PMID: 24723564 Free PMC article. Review.
Cited by
-
Denosumab combined with chemotherapy followed by anlotinib in the treatment of multiple metastases of malignant peripheral nerve sheath tumor: a case report and literature review.Front Oncol. 2024 Jul 25;14:1399021. doi: 10.3389/fonc.2024.1399021. eCollection 2024. Front Oncol. 2024. PMID: 39119091 Free PMC article.
-
Malignant peripheral nerve sheath tumor with hemophilic syndrome and bone marrow fibrosis: A rare case report.World J Clin Cases. 2023 Nov 6;11(31):7673-7679. doi: 10.12998/wjcc.v11.i31.7673. World J Clin Cases. 2023. PMID: 38078124 Free PMC article.
-
Anthracyclines as Topoisomerase II Poisons: From Early Studies to New Perspectives.Int J Mol Sci. 2018 Nov 6;19(11):3480. doi: 10.3390/ijms19113480. Int J Mol Sci. 2018. PMID: 30404148 Free PMC article. Review.
-
The IL13α 2R paves the way for anti-glioma nanotherapy.Genes Dis. 2021 Sep 15;10(1):89-100. doi: 10.1016/j.gendis.2021.08.006. eCollection 2023 Jan. Genes Dis. 2021. PMID: 37013057 Free PMC article. Review.
References
-
- Ferrari A, Bisogno G, Macaluso A, Casanova M, D'Angelo P, Pierani P, et al. Soft-tissue sarcomas in children and adolescents with neurofibromatosis type 1. Cancer. 2007;109(7):1406–12. doi: 10.1002/cncr.22533 . - DOI - PubMed
-
- Park MK, Sung JK, Nam KH, Kim KT. Malignant peripheral nerve sheath tumor of non-neurofibromatosis type I metastasized to the cerebrospinal axis. J Korean Neurosurg Soc. 2013;53(3):190–3. doi: 10.3340/jkns.2013.53.3.190 ; PubMed Central PMCID: PMC3638275. - DOI - PMC - PubMed
-
- Sheikh OA, Reaves A, Kralick FA, Brooks A, Musial RE, Gasperino J. Malignant nerve sheath tumor of the spinal accessory nerve: a unique presentation of a rare tumor. J Clin Neurol. 2012;8(1):75–8. doi: 10.3988/jcn.2012.8.1.75 ; PubMed Central PMCID: PMC3325436. - DOI - PMC - PubMed
-
- Kitamura M, Wada N, Nagata S, Iizuka N, Jin YF, Tomoeda M, et al. Malignant peripheral nerve sheath tumor associated with neurofibromatosis type 1, with metastasis to the heart: a case report. Diagn Pathol. 2010;5:2 Epub 2010/03/09. doi: 10.1186/1746-1596-5-2 ; PubMed Central PMCID: PMC2881068. - DOI - PMC - PubMed
-
- Park SK, Yi HJ, Paik SS, Kim YJ, Ko Y, Oh SJ. Metastasizing malignant peripheral nerve sheath tumor initially presenting as intracerebral hemorrhage. Case report and review of the literature. Surg Neurol. 2007;68(1):79–84; doi: 10.1016/j.surneu.2006.10.033 . - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

