ERK inhibition promotes neuroectodermal precursor commitment by blocking self-renewal and primitive streak formation of the epiblast
- PMID: 29304842
- PMCID: PMC5756365
- DOI: 10.1186/s13287-017-0750-8
ERK inhibition promotes neuroectodermal precursor commitment by blocking self-renewal and primitive streak formation of the epiblast
Abstract
Background: Pluripotent stem cells hold great promise for regenerative medicine. However, before clinical application, reproducible protocols for pluripotent stem cell differentiation should be established. Extracellular signal-regulated protein kinase (ERK) signaling plays a central role for the self-renewal of epiblast stem cells (EpiSCs), but its role for subsequent germ layer differentiation is still ambiguous. We proposed that ERK could modulate differentiation of the epiblast.
Methods: PD0325901 was used to inhibit ERK activation during the differentiation of embryonic stem cells and EpiSCs. Immunofluorescence, western blot analysis, real-time PCR and flow cytometry were used to detect germ layer markers and pathway activation.
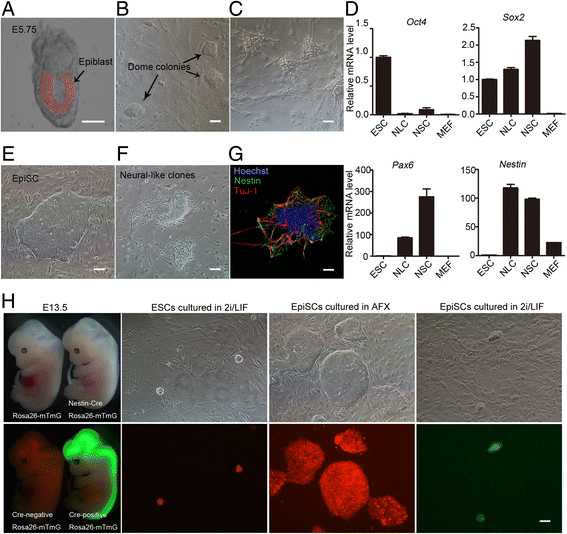
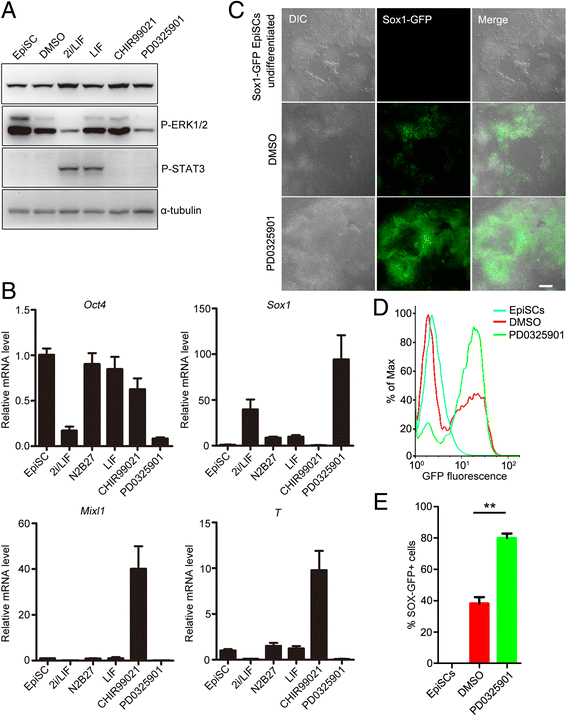
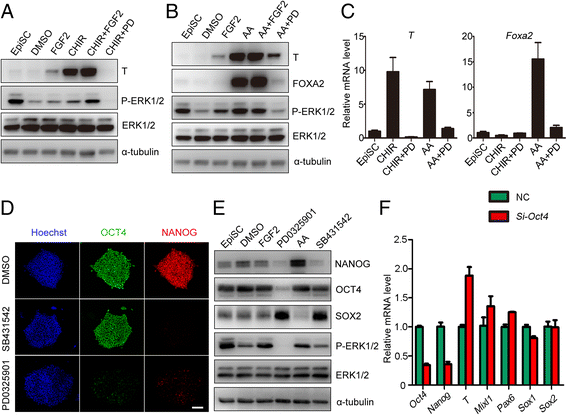
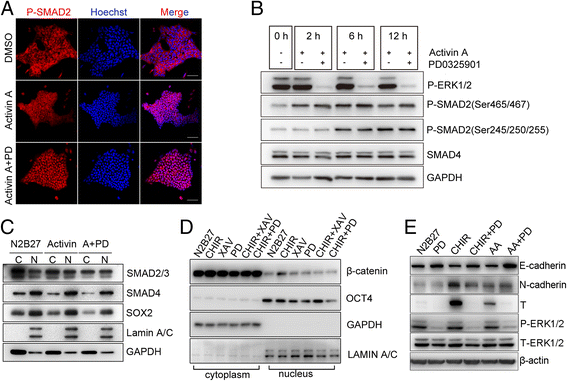
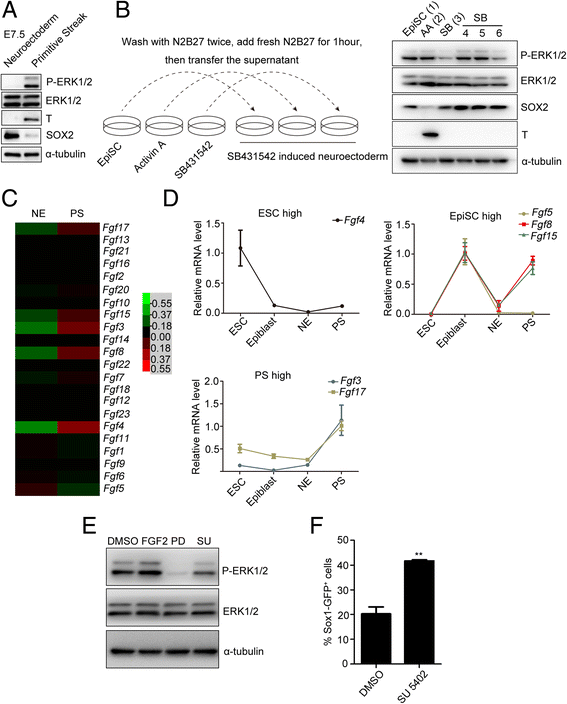
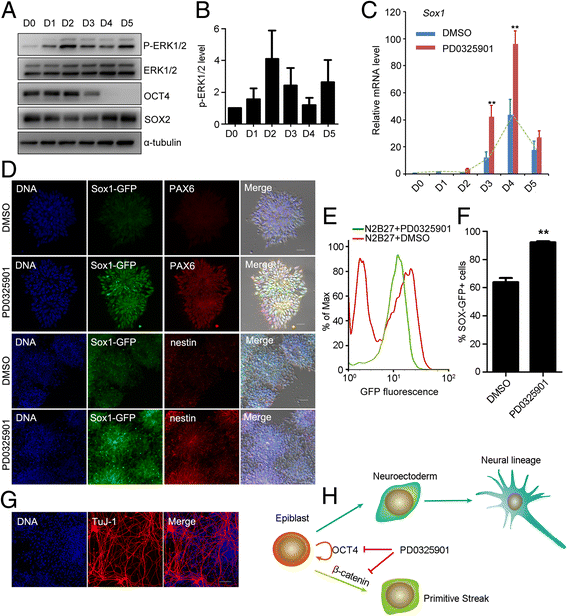
Results: We demonstrate that the ERK phosphorylation level is lower in neuroectoderm of mouse E7.5 embryos than that in the primitive streak. ERK inhibition results in neural lineage commitment of epiblast. Mechanistically, PD0325901 abrogates the expression of primitive streak markers by β-catenin retention in the cytoplasm, and inhibits the expression of OCT4 and NANOG during EpiSC differentiation. Thus, EpiSCs differentiate into neuroectodermal lineage efficiently under PD0325901 treatment. These results suggest that neuroectoderm differentiation does not require extrinsic signals, supporting the default differentiation of neural lineage.
Conclusions: We report that a single ERK inhibitor, PD0325901, can specify epiblasts and EpiSCs into neural-like cells, providing an efficient strategy for neural differentiation.
Keywords: Embryonic stem cells; Epiblast stem cells; Extracellular signal-regulated protein kinase; Neural differentiation; PD0325901.
Conflict of interest statement
Ethics approval and consent to participate
Animal experiments were conducted in accordance with the guidelines of the Institutional Animal Care and Use Committee at Institute of Zoology, Chinese Academy of Sciences.
Consent for publication
All authors have contributed to, read, and approved the final manuscript for submission.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures
Similar articles
-
Distinct Wnt-driven primitive streak-like populations reflect in vivo lineage precursors.Development. 2014 Mar;141(6):1209-21. doi: 10.1242/dev.101014. Development. 2014. PMID: 24595287 Free PMC article.
-
The transcriptional and functional properties of mouse epiblast stem cells resemble the anterior primitive streak.Cell Stem Cell. 2014 Jan 2;14(1):107-20. doi: 10.1016/j.stem.2013.09.014. Epub 2013 Oct 17. Cell Stem Cell. 2014. PMID: 24139757
-
Characterization of Oct4-GFP spermatogonial stem cell line and its application in the reprogramming studies.J Cell Biochem. 2013 Apr;114(4):920-8. doi: 10.1002/jcb.24431. J Cell Biochem. 2013. PMID: 23097321
-
Heterogeneity in Epiblast Stem Cells.Adv Exp Med Biol. 2019;1123:5-17. doi: 10.1007/978-3-030-11096-3_2. Adv Exp Med Biol. 2019. PMID: 31016592 Review.
-
Generating primed pluripotent epiblast stem cells: A methodology chapter.Curr Top Dev Biol. 2020;138:139-174. doi: 10.1016/bs.ctdb.2020.01.005. Epub 2020 Feb 27. Curr Top Dev Biol. 2020. PMID: 32220296 Free PMC article. Review.
Cited by
-
A guide to ERK dynamics, part 2: downstream decoding.Biochem J. 2023 Dec 13;480(23):1909-1928. doi: 10.1042/BCJ20230277. Biochem J. 2023. PMID: 38038975 Free PMC article.
-
Inhibition of MEK1/2 Signaling Pathway Limits M2 Macrophage Polarization and Interferes in the Dental Socket Repair Process in Mice.Biology (Basel). 2025 Jan 21;14(2):107. doi: 10.3390/biology14020107. Biology (Basel). 2025. PMID: 40001875 Free PMC article.
-
Modeling Mammalian Commitment to the Neural Lineage Using Embryos and Embryonic Stem Cells.Front Physiol. 2019 Jul 11;10:705. doi: 10.3389/fphys.2019.00705. eCollection 2019. Front Physiol. 2019. PMID: 31354503 Free PMC article. Review.
-
Nuclear FAM289-Galectin-1 interaction controls FAM289-mediated tumor promotion in malignant glioma.J Exp Clin Cancer Res. 2019 Sep 6;38(1):394. doi: 10.1186/s13046-019-1393-7. J Exp Clin Cancer Res. 2019. PMID: 31492191 Free PMC article.
-
The functional diversity of the POUV-class proteins across vertebrates.Open Biol. 2022 Jun;12(6):220065. doi: 10.1098/rsob.220065. Epub 2022 Jun 29. Open Biol. 2022. PMID: 35765816 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

