Effectiveness and safety of ferric carboxymaltose compared to iron sucrose in women with iron deficiency anemia: phase IV clinical trials
- PMID: 29304848
- PMCID: PMC5755312
- DOI: 10.1186/s12905-017-0506-8
Effectiveness and safety of ferric carboxymaltose compared to iron sucrose in women with iron deficiency anemia: phase IV clinical trials
Abstract
Background: Iron deficiency anemia (IDA) is a significant problem worldwide particularly in women. The aim of the study was to evaluate the effectiveness and safety of intravenous ferric carboxymaltose (FCM) in comparison to iron sucrose (IS) in women with IDA.
Method: Two hundred patients at Department of Obstetrics and Gynaecology, Sher-i-Kashmir Institute of Medical Sciences Medical College and Hospital, Jammu & Kashmir, India identified with IDA were enrolled for the study. Intravenous FCM and IS were both given as per the protocol. Change in the laboratory parameters such as hemoglobin (Hb), mean corpuscular value, and serum ferritin levels at two weeks and four weeks interval after the treatment was recorded.
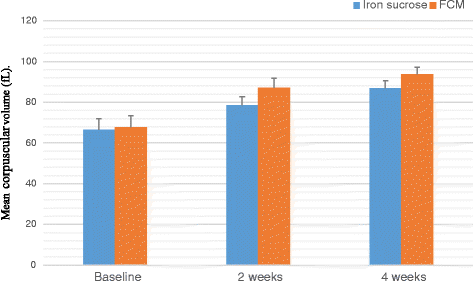
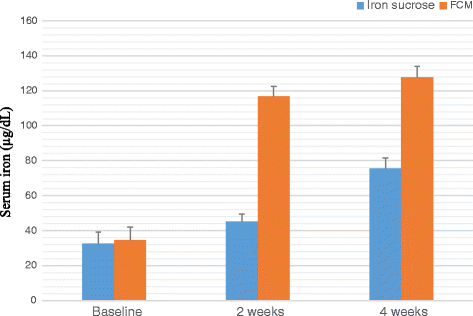
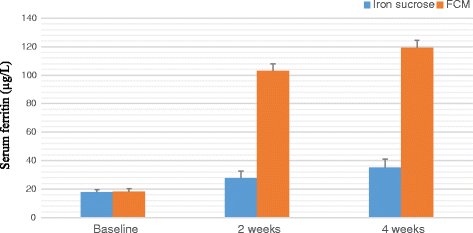
Result: A significant increase in the mean Hb was observed from 7.76 ± 0.709 to 13.25 ± 0.606 in patients treated with FCM and 7.64 ± 0.710 to 11.59 ± 0.733 g/dL (P < 0.001) in patients treated with IS after four weeks of therapy. The rise in mean corpuscular volume was from 66.82 ± 5.24 to 86.76 ± 3.765 and 68.05 ± 5.56 to 93.80 ± 3.80 and rise in serum ferritin levels were from 8.32 ± 1.787 to 38.94 ± 6.095 μg/L and 8.16 ± 1.540 to 27 ± 8.175 μg/L in patients treated with FCM and IS respectively after four weeks of therapy. No serious adverse effects were reported.
Conclusion: Parenteral therapy is effective in IDA, but FCM elevates hemoglobin level and restored iron stores faster than IS with minimum adverse drug reactions.
Trial registration number: ISRCTN14484575 Dated: 15-12-2017 retrospectively registered. https://doi.org/10.1186/ISRCTN14484575.
Keywords: Ferric carboxymaltose; Hemoglobin; Iron deficiency anemia; Iron sucrose; Serum ferritin.
Conflict of interest statement
Ethics approval and consent to participate
Institutional Ethics Committee Sher-i-Kashmir Institute of Medical Sciences Medical College and Hospital (IEC - SKIMS MCH), in accordance with Indian Council of Medical Research (ICMR) guidelines. Institutional Ethical Registration Number: SKIMS MC/CM/IEC/15/42–50. Dated 16–07-2015.
Written as well as verbal consent was taken by each participant.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures




References
-
- Glazer Y, Bilenko N. Effect of iron deficiency and iron deficiency anemia in the first two years of life on cognitive and mental development during childhood. Harefuah. 2010;149(5):309–314,335. - PubMed
-
- WHO. Nutritional anemias. Report of a WHO scientific group. Geneva, World Health Organization, 1968. (WHO Technical Report Series, No. 405). http://apps.who.int/iris/bitstream/10665/40707/1/WHO_TRS_405.pdf. Accessed 5 Nov 2017. - PubMed
-
- WHO/UNICEF/UNU. Iron deficiency anaemia assessment, prevention, and control: a guide for programme managers. Geneva, World Health Organization, 2001. Available at: http://www.who.int/nutrition/publications/micronutrients/anaemia_iron_de.... Accessed 5 Nov 2017.
-
- Global Burden of Disease Study 2013, Collaborators Global, regional, and national incidence, prevalence, and years lived with disability for 301 acute and chronic diseases and injuries in 188 countries, 1990-2013: a systematic analysis for the global burden of disease study 2013. Lancet. 2015;386(9995):743–800. doi: 10.1016/S0140-6736(15)60692-4. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources

