Is ABCA1 a lipid transfer protein?
- PMID: 29305383
- PMCID: PMC5928442
- DOI: 10.1194/jlr.R082313
Is ABCA1 a lipid transfer protein?
Abstract
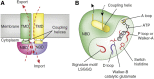
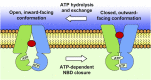
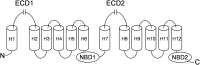
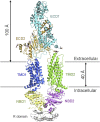
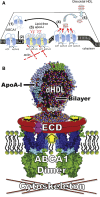
ABCA1 functions as a lipid transporter because it mediates the transfer of cellular phospholipid (PL) and free (unesterified) cholesterol (FC) to apoA-I and related proteins present in the extracellular medium. ABCA1 is a membrane PL translocase and its enzymatic activity leads to transfer of PL molecules from the cytoplasmic leaflet to the exofacial leaflet of a cell plasma membrane (PM). The presence of active ABCA1 in the PM promotes binding of apoA-I to the cell surface. About 10% of this bound apoA-I interacts directly with ABCA1 and stabilizes the transporter. Most of the pool of cell surface-associated apoA-I is bound to lipid domains in the PM that are created by the activity of ABCA1. The amphipathic α-helices in apoA-I confer detergent-like properties on the protein enabling it to solubilize PL and FC in these membrane domains to create a heterogeneous population of discoidal nascent HDL particles. This review focuses on current understanding of the structure-function relationships of human ABCA1 and the molecular mechanisms underlying HDL particle production.
Keywords: ATP binding cassette transporter A1; apolipoprotein A-I; cholesterol; high density lipoprotein; phospholipid.
Copyright © 2018 by the American Society for Biochemistry and Molecular Biology, Inc.
Figures


References
-
- Oram J. F. 2000. Tangier disease and ABCA1. Biochim. Biophys. Acta. 1529: 321–330. - PubMed
-
- Timmins J. M., Lee J. Y., Boudyguina E., Kluckman K. D., Brunham L. R., Mulya A., Gebre A. K., Coutinho J. M., Colvin P. L., Smith T. L., et al. . 2005. Targeted inactivation of hepatic Abca1 causes profound hypoalphalipoproteinemia and kidney hypercatabolism of apoA-I. J. Clin. Invest. 115: 1333–1342. - PMC - PubMed
-
- Oram J. F., and Heinecke J. W.. 2005. ATP-binding cassette transporter A1: a cell cholesterol exporter that protects against cardiovascular disease. Physiol. Rev. 85: 1343–1372. - PubMed
-
- Yokoyama S. 2006. Assembly of high-density lipoprotein. Arterioscler. Thromb. Vasc. Biol. 26: 20–27. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

