Tumor stressors induce two mechanisms of intracellular P-glycoprotein-mediated resistance that are overcome by lysosomal-targeted thiosemicarbazones
- PMID: 29305422
- PMCID: PMC5846167
- DOI: 10.1074/jbc.M116.772699
Tumor stressors induce two mechanisms of intracellular P-glycoprotein-mediated resistance that are overcome by lysosomal-targeted thiosemicarbazones
Abstract
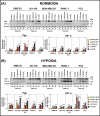
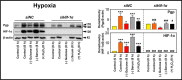
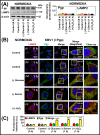
Multidrug resistance (MDR) is a major obstacle in cancer treatment due to the ability of tumor cells to efflux chemotherapeutics via drug transporters (e.g. P-glycoprotein (Pgp; ABCB1)). Although the mechanism of Pgp-mediated drug efflux is known at the plasma membrane, the functional role of intracellular Pgp is unclear. Moreover, there has been intense focus on the tumor micro-environment as a target for cancer treatment. This investigation aimed to dissect the effects of tumor micro-environmental stress on subcellular Pgp expression, localization, and its role in MDR. These studies demonstrated that tumor micro-environment stressors (i.e. nutrient starvation, low glucose levels, reactive oxygen species, and hypoxia) induce Pgp-mediated drug resistance. This occurred by two mechanisms, where stressors induced 1) rapid Pgp internalization and redistribution via intracellular trafficking (within 1 h) and 2) hypoxia-inducible factor-1α expression after longer incubations (4-24 h), which up-regulated Pgp and was accompanied by lysosomal biogenesis. These two mechanisms increased lysosomal Pgp and facilitated lysosomal accumulation of the Pgp substrate, doxorubicin, resulting in resistance. This was consistent with lysosomal Pgp being capable of transporting substrates into lysosomes. Hence, tumor micro-environmental stressors result in: 1) Pgp redistribution to lysosomes; 2) increased Pgp expression; 3) lysosomal biogenesis; and 4) potentiation of Pgp substrate transport into lysosomes. In contrast to doxorubicin, when stress stimuli increased lysosomal accumulation of the cytotoxic Pgp substrate, di-2-pyridylketone 4,4-dimethyl-3-thiosemicarbazone (Dp44mT), this resulted in the agent overcoming resistance. Overall, this investigation describes a novel approach to overcoming resistance in the stressful tumor micro-environment.
Keywords: ABCB1; P-glycoprotein; drug delivery; drug resistance; drug transport; lysosome; tumor micro-environment; tumor micro-environmental stress.
© 2018 by The American Society for Biochemistry and Molecular Biology, Inc.
Conflict of interest statement
D. R. R. is a stakeholder in Oncochel Therapeutics LLC and Pty Ltd, which is developing the thiosemicarbazone, DpC, for the treatment of advanced and resistant cancer
Figures




Similar articles
-
Di-2-pyridylketone 4,4-dimethyl-3-thiosemicarbazone (Dp44mT) overcomes multidrug resistance by a novel mechanism involving the hijacking of lysosomal P-glycoprotein (Pgp).J Biol Chem. 2015 Apr 10;290(15):9588-603. doi: 10.1074/jbc.M114.631283. Epub 2015 Feb 26. J Biol Chem. 2015. PMID: 25720491 Free PMC article.
-
Glucose Modulation Induces Lysosome Formation and Increases Lysosomotropic Drug Sequestration via the P-Glycoprotein Drug Transporter.J Biol Chem. 2016 Feb 19;291(8):3796-820. doi: 10.1074/jbc.M115.682450. Epub 2015 Nov 24. J Biol Chem. 2016. PMID: 26601947 Free PMC article.
-
A mechanism for overcoming P-glycoprotein-mediated drug resistance: novel combination therapy that releases stored doxorubicin from lysosomes via lysosomal permeabilization using Dp44mT or DpC.Cell Death Dis. 2016 Dec 1;7(12):e2510. doi: 10.1038/cddis.2016.381. Cell Death Dis. 2016. PMID: 27906178 Free PMC article.
-
Turning the gun on cancer: Utilizing lysosomal P-glycoprotein as a new strategy to overcome multi-drug resistance.Free Radic Biol Med. 2016 Jul;96:432-45. doi: 10.1016/j.freeradbiomed.2016.04.201. Epub 2016 May 3. Free Radic Biol Med. 2016. PMID: 27154979 Review.
-
Multidrug resistance-associated protein: a protein distinct from P-glycoprotein involved in cytotoxic drug expulsion.Gen Pharmacol. 1997 May;28(5):639-45. doi: 10.1016/s0306-3623(96)00284-4. Gen Pharmacol. 1997. PMID: 9184795 Review.
Cited by
-
Is P-Glycoprotein Functionally Expressed in the Limiting Membrane of Endolysosomes? A Biochemical and Ultrastructural Study in the Rat Liver.Cells. 2022 May 5;11(9):1556. doi: 10.3390/cells11091556. Cells. 2022. PMID: 35563868 Free PMC article.
-
Advances in the structure, mechanism and targeting of chemoresistance-linked ABC transporters.Nat Rev Cancer. 2023 Nov;23(11):762-779. doi: 10.1038/s41568-023-00612-3. Epub 2023 Sep 15. Nat Rev Cancer. 2023. PMID: 37714963 Review.
-
Synthesis and Biological Evaluation of Seco-Coumarin/Furoxan Hybrids as Potent Anti-Tumor Agents to Overcome Multidrug Resistance via Multiple Mechanisms.Molecules. 2025 May 27;30(11):2341. doi: 10.3390/molecules30112341. Molecules. 2025. PMID: 40509228 Free PMC article.
-
Role of lysosomes in physiological activities, diseases, and therapy.J Hematol Oncol. 2021 May 14;14(1):79. doi: 10.1186/s13045-021-01087-1. J Hematol Oncol. 2021. PMID: 33990205 Free PMC article. Review.
-
Unveiling the mechanisms and challenges of cancer drug resistance.Cell Commun Signal. 2024 Feb 12;22(1):109. doi: 10.1186/s12964-023-01302-1. Cell Commun Signal. 2024. PMID: 38347575 Free PMC article. Review.
References
-
- Germann U. A. (1996) P-glycoprotein: a mediator of multidrug resistance in tumour cells. Eur. J. Cancer 32A, 927–944 - PubMed
-
- Cole S. P., Bhardwaj G., Gerlach J. H., Mackie J. E., Grant C. E., Almquist K. C., Stewart A. J., Kurz E. U., Duncan A. M., and Deeley R. G. (1992) Overexpression of a transporter gene in a multidrug-resistant human lung cancer cell line. Science 258, 1650–1654 10.1126/science.1360704 - DOI - PubMed
-
- Comerford K. M., Wallace T. J., Karhausen J., Louis N. A., Montalto M. C., and Colgan S. P. (2002) Hypoxia-inducible factor-1-dependent regulation of the multidrug resistance (MDR1) gene. Cancer Res. 62, 3387–3394 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

