Impaired lymphoid extracellular matrix impedes antibacterial immunity in epidermolysis bullosa
- PMID: 29305555
- PMCID: PMC5789908
- DOI: 10.1073/pnas.1709111115
Impaired lymphoid extracellular matrix impedes antibacterial immunity in epidermolysis bullosa
Abstract
Genetic loss of collagen VII causes recessive dystrophic epidermolysis bullosa (RDEB), a skin fragility disorder that, unexpectedly, manifests also with elevated colonization of commensal bacteria and frequent wound infections. Here, we describe an unprecedented systemic function of collagen VII as a member of a unique innate immune-supporting multiprotein complex in spleen and lymph nodes. In this complex, collagen VII specifically binds and sequesters the innate immune activator cochlin in the lumen of lymphoid conduits. In genetic mouse models, loss of collagen VII increased bacterial colonization by diminishing levels of circulating cochlin LCCL domain. Intraperitoneal injection of collagen VII, which restored cochlin in the spleen, but not in the skin, reactivated peripheral innate immune cells via cochlin and reduced bacterial skin colonization. Systemic administration of the cochlin LCCL domain was alone sufficient to diminish bacterial supercolonization of RDEB mouse skin. Human validation demonstrated that RDEB patients displayed lower levels of systemic cochlin LCCL domain with subsequently impaired macrophage response in infected wounds. This study identifies an intrinsic innate immune dysfunction in RDEB and uncovers a unique role of the lymphoid extracellular matrix in systemic defense against bacteria.
Keywords: bacteria; cochlin; collagen VII; innate immunity; recessive dystrophic epidermolysis bullosa.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

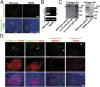
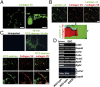

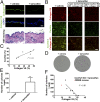



References
-
- Guerra L, Odorisio T, Zambruno G, Castiglia D. Stromal microenvironment in type VII collagen-deficient skin: The ground for squamous cell carcinoma development. Matrix Biol. 2017;63:1–10. - PubMed
-
- Fine J-D, Johnson LB, Weiner M, Li K-P, Suchindran C. Epidermolysis bullosa and the risk of life-threatening cancers: The National EB Registry experience, 1986-2006. J Am Acad Dermatol. 2009;60:203–211. - PubMed
-
- Mittapalli VR, et al. Injury-driven stiffening of the dermis expedites skin carcinoma progression. Cancer Res. 2016;76:940–951. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

