Effects of Weight-Loss Medications on Cardiometabolic Risk Profiles: A Systematic Review and Network Meta-analysis
- PMID: 29305933
- PMCID: PMC5880739
- DOI: 10.1053/j.gastro.2017.12.024
Effects of Weight-Loss Medications on Cardiometabolic Risk Profiles: A Systematic Review and Network Meta-analysis
Abstract
Background & aims: We performed a systematic review and network meta-analysis to evaluate the overall and comparative effects of weight-loss medications approved by the Food and Drug Administration for long-term use on cardiometabolic risk profiles of obese adults.
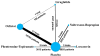
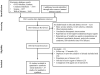
Methods: We performed a systematic literature review through February 28, 2017 to identify randomized clinical trials of the effects of Food and Drug Administration-approved weight-loss medications (ie, orlistat, lorcaserin, naltrexone-bupropion, phentermine-topiramate, and liraglutide) administered to obese adults for 1 year or more, compared with placebo or another active agent. Outcomes of interest included changes in blood glucose (fasting blood glucose [FBG] and hemoglobin A1c), cholesterol profile (low-density lipoprotein and high-density lipoproteins), blood pressure (BP; systolic/diastolic), and waist circumference (WC). We performed pair-wise and network meta-analyses with outcomes reported as weighted and standardized mean differences. Quality of evidence was rated using GRADE (Grading of Recommendations Assessment, Development and Evaluation).
Results: In a meta-analysis of 28 randomized controlled trials (29,018 participants; median body mass index, 36.1 kg/m2), we associated weight-loss medications with a modest decrease in FBG (weighted mean difference, 4.0 mg/dL; 95% confidence interval, -4.4 to -3.6 mg/dL) and WC (weighted mean difference, reduction of 3.3 cm; 95% confidence interval, -3.5 to -3.1 cm), without clinically meaningful changes in systolic/diastolic BP or cholesterol profile vs placebo (standardized mean difference <0.2); effects varied among drugs. Phentermine-topiramate use was associated with a substantial decrease in WC and a modest decrease in FBG, hemoglobin A1c, and BP, and had minimal effect on cholesterol. Liraglutide use was associated with a substantial decrease in FBG, hemoglobin A1c, and WC, and a minimal effect on BP and cholesterol. Naltrexone-bupropion use was associated with moderate increase in high-density lipoprotein cholesterol, but had a minimal effect on FBG and WC. Orlistat use was associated with a decrease in low-density lipoprotein and high-density lipoprotein cholesterol. No drug improved all cardiometabolic risk factors.
Conclusions: In a systematic review and network meta-analysis, we found Food and Drug Administration-approved weight-loss medications to have only modest positive effects on cardiometabolic risk profile. Further research is needed to evaluate the long-term cardiometabolic benefits of these medications.
Prospero: CRD42016039486.
Keywords: BMI; Heart Disease; Pharmacotherapy; Vascular.
Copyright © 2018 AGA Institute. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Figures


References
-
- Jensen MD, Ryan DH, Apovian CM, et al. 2013 AHA/ACC/TOS guideline for the management of overweight and obesity in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and The Obesity Society. Circulation. 2014;129(25 Suppl 2):S102–138. - PMC - PubMed
-
- Curioni CC, Lourenco PM. Long-term weight loss after diet and exercise: a systematic review. Int J Obes (Lond) 2005;29(10):1168–1174. - PubMed
-
- Nissen SE, Wolski KE, Prcela L, et al. Effect of Naltrexone-Bupropion on Major Adverse Cardiovascular Events in Overweight and Obese Patients With Cardiovascular Risk Factors: A Randomized Clinical Trial. JAMA. 2016;315(10):990–1004. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

