Safety and Effectiveness of Ledipasvir and Sofosbuvir, With or Without Ribavirin, in Treatment-Experienced Patients With Genotype 1 Hepatitis C Virus Infection and Cirrhosis
- PMID: 29306043
- PMCID: PMC6034985
- DOI: 10.1016/j.cgh.2017.12.037
Safety and Effectiveness of Ledipasvir and Sofosbuvir, With or Without Ribavirin, in Treatment-Experienced Patients With Genotype 1 Hepatitis C Virus Infection and Cirrhosis
Abstract
Background & aims: We aimed to evaluate the safety and effectiveness of 12 or 24 weeks treatment with ledipasvir and sofosbuvir, with or without ribavirin, in treatment-experienced patients with hepatitis C virus (HCV) genotype 1 infection and cirrhosis in routine clinical practice. Patients were followed in a multi-center, prospective, observational cohort study (HCV-TARGET).
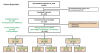
Methods: We collected data from 667 treatment-experienced adults with chronic genotype 1 HCV infection who began treatment with ledipasvir and sofosbuvir, with or without ribavirin, from 2011 through September 15, 2016, according to the regional standards of care, at academic (n = 39) and community (n = 18) centers in the United States, Canada, Germany, and Israel. Information was collected from medical records and abstracted into a unique centralized data core. Independent monitors systematically reviewed data entries for completeness and accuracy. Demographic, clinical, adverse event, and virologic data were collected every 12 weeks during treatment and during the follow-up period. The primary efficacy endpoint was sustained virologic response, defined as a level of HCV RNA below the lower limit of quantification or undetectable at a minimum 64 days after the end of treatment (SVR12). The per-protocol population (n = 610) was restricted to patients who completed 12 or 24 weeks of treatment (±2 weeks) and had final virologic outcomes available.
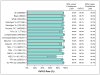
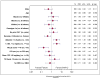
Results: The per-protocol analysis revealed that 579 patients (93.8%) achieved an SVR12, including 50/51 patients who received ledipasvir and sofosbuvir for 12 weeks (98%), 384/408 patients who received ledipasvir and sofosbuvir for 24 weeks (94.1%), 68/70 patients who received ledipasvir and sofosbuvir with ribavirin for 12 weeks (97.1%), and 57/60 patients who received ledipasvir and sofosbuvir with ribavirin for 24 weeks (95%). On multivariate analysis, neither treatment duration nor the addition of ribavirin was associated with SVR12. Compensated cirrhosis (odds ratio [OR] compared to decompensated cirrhosis, 2.41; 95% CI, 1.16-5.02), albumin ≥ 3.5 g/dL (OR, 3.15; 95% CI 1.46-6.80), or total bilirubin ≤ 1.2 mg/dL (OR 3.34; 95% CI, 1.59-7.00) were associated with SVR12.
Conclusions: In an analysis of safety and effectiveness data from the HCV-TARGET study, we found treatment with ledipasvir and sofosbuvir, with or without ribavirin, to be effective and well tolerated by treatment-experienced patients with genotype 1 HCV infection and compensated cirrhosis. There were no significant differences in rate of SVR12 among patients treated with ledipasvir and sofosbuvir for 12 or 24 weeks, with or without ribavirin. Patients with decompensated cirrhosis appear to benefit from the addition of ribavirin or extension of ledipasvir and sofosbuvir treatment to 24 weeks. ClinicalTrials.gov no: NCT10474811.
Keywords: HCV-TARGET; Liver Disease; Real World; Therapy; Viral Hepatitis.
Copyright © 2018 AGA Institute. Published by Elsevier Inc. All rights reserved.
Figures
Similar articles
-
Effectiveness of Ledipasvir-Sofosbuvir Combination in Patients With Hepatitis C Virus Infection and Factors Associated With Sustained Virologic Response.Gastroenterology. 2016 Dec;151(6):1131-1140.e5. doi: 10.1053/j.gastro.2016.08.004. Epub 2016 Aug 24. Gastroenterology. 2016. PMID: 27565882 Free PMC article.
-
Ledipasvir and sofosbuvir fixed-dose combination with and without ribavirin for 12 weeks in treatment-naive and previously treated Japanese patients with genotype 1 hepatitis C: an open-label, randomised, phase 3 trial.Lancet Infect Dis. 2015 Jun;15(6):645-53. doi: 10.1016/S1473-3099(15)70099-X. Epub 2015 Apr 8. Lancet Infect Dis. 2015. PMID: 25863559 Clinical Trial.
-
Efficacy of ledipasvir and sofosbuvir, with or without ribavirin, for 12 weeks in patients with HCV genotype 3 or 6 infection.Gastroenterology. 2015 Nov;149(6):1454-1461.e1. doi: 10.1053/j.gastro.2015.07.063. Epub 2015 Aug 7. Gastroenterology. 2015. PMID: 26261007 Clinical Trial.
-
Ledipasvir-sofosbuvir: interferon-/ribavirin-free regimen for chronic hepatitis C virus infection.Ann Pharmacother. 2015 Mar;49(3):343-50. doi: 10.1177/1060028014563952. Epub 2014 Dec 16. Ann Pharmacother. 2015. PMID: 25515863 Review.
-
Ledipasvir/sofosbuvir with or without ribavirin for the treatment of chronic hepatitis C genotype 1: A pairwise meta-analysis.J Gastroenterol Hepatol. 2017 Apr;32(4):749-755. doi: 10.1111/jgh.13620. J Gastroenterol Hepatol. 2017. PMID: 27785825
Cited by
-
Initial uptake, time to treatment, and real-world effectiveness of all-oral direct-acting antivirals for hepatitis C virus infection in the United States: A retrospective cohort analysis.PLoS One. 2019 Aug 22;14(8):e0218759. doi: 10.1371/journal.pone.0218759. eCollection 2019. PLoS One. 2019. PMID: 31437170 Free PMC article.
-
Viral Hepatitis C Therapy: Pharmacokinetic and Pharmacodynamic Considerations: A 2019 Update.Clin Pharmacokinet. 2019 Oct;58(10):1237-1263. doi: 10.1007/s40262-019-00774-0. Clin Pharmacokinet. 2019. PMID: 31114957 Free PMC article. Review.
-
Managing HBV and HCV Infection Pre- and Post-liver Transplant.J Clin Exp Hepatol. 2024 Mar-Apr;14(2):101287. doi: 10.1016/j.jceh.2023.09.008. Epub 2023 Sep 23. J Clin Exp Hepatol. 2024. PMID: 38076445 Free PMC article. Review.
-
Expanding Hepatitis C Virus Care and Cure: National Experience Using a Clinical Pharmacist-Driven Model.Open Forum Infect Dis. 2019 Jul 1;6(7):ofz316. doi: 10.1093/ofid/ofz316. Open Forum Infect Dis. 2019. PMID: 31363775 Free PMC article.
-
Economic evaluation of pan-genotypic generic direct-acting antiviral regimens for treatment of chronic hepatitis C in Iran: a cost-effectiveness study.BMJ Open. 2022 Jun 8;12(6):e058757. doi: 10.1136/bmjopen-2021-058757. BMJ Open. 2022. PMID: 35676019 Free PMC article.
References
-
- Blach S, Zeuzem S, Manns M, et al. Global prevalence and genotype distribution of hepatitis C virus infection in 2015: a modelling study. Lancet Gastroenterol Hepatol. 2017;2:161–176. - PubMed
-
- Westbrook RH, Dusheiko G. Natural history of hepatitis C. J Hepatol. 2014;61(1 Suppl):S58–68. - PubMed
-
- van der Meer AJ, Wedemeyer H, Feld JJ, et al. Life expectancy in patients with chronic HCV infection and cirrhosis compared with a general population. JAMA. 2014;312(18):1927–8. - PubMed
-
- van der Meer AJ, Veldt BJ, Feld JJ, et al. Association between sustained virological response and all-cause mortality among patients with chronic hepatitis C and advanced hepatic fibrosis. JAMA. 2012;308(24):2584–93. - PubMed
-
- Gower E, Estes C, Blach S, et al. Global epidemiology and genotype distribution of the hepatitis C virus infection. J Hepatol. 2014;61(1 Suppl):S45–57. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

