Mechanical stress potentiates the differentiation of periodontal ligament stem cells into keratocytes
- PMID: 29306866
- PMCID: PMC5890647
- DOI: 10.1136/bjophthalmol-2017-311150
Mechanical stress potentiates the differentiation of periodontal ligament stem cells into keratocytes
Abstract
Aims: To explore the role of corneal-shaped static mechanical strain on the differentiation of human periodontal ligament stem cells (PDLSCs) into keratocytes and the possible synergistic effects of mechanics and inducing medium.
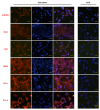
Methods: PDLSCs were exposed to 3% static dome-shaped mechanical strain in a Flexcell Tension System for 3 days and 7 days. Keratocyte phenotype was determined by gene expression of keratocyte markers. Keratocyte differentiation (inducing) medium was introduced in the Flexcell system, either continuously or intermittently combined with mechanical stimulation. The synergistic effects of mechanics and inducing medium on keratocyte differentiation was evaluated by gene and protein expression of keratocyte markers. Finally, a multilamellar cell sheet was assembled by seeding PDLSCs on a collagen membrane and inducing keratocyte differentiation. The transparency of the cell sheet was assessed, and typical markers of native human corneal stroma were evaluated by immunofluorescence staining.
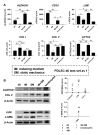
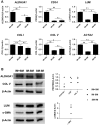
Results: Dome-shaped mechanical stimulation promoted PDLSCs to differentiate into keratocytes, as shown by the upregulation of ALDH3A1, CD34, LUM, COL I and COL V. The expression of integrins were also upregulated after mechanical stimulation, including integrin alpha 1, alpha 2, beta 1 and non-muscle myosin II B. A synergistic effect of mechanics and inducing medium was found on keratocyte differentiation. The cell sheets were assembled under the treatment of mechanics and inducing medium simultaneously. The cell sheets were transparent, multilamellar and expressed typical markers of corneal stroma.
Conclusion: Dome-shaped mechanical stimulation promotes differentiation of PDLSCs into keratocytes and has synergistic effects with inducing medium. Multilamellar cell sheets that resemble native human corneal stroma show potential for future clinical applications.
Keywords: PDLSCs; cell-sheet; corneal stroma; differentiation; inducing medium; mechanics.
© Article author(s) (or their employer(s) unless otherwise stated in the text of the article) 2018. All rights reserved. No commercial use is permitted unless otherwise expressly granted.
Conflict of interest statement
Competing interests: None declared.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous
