Patterns and predictors of skin score change in early diffuse systemic sclerosis from the European Scleroderma Observational Study
- PMID: 29306872
- PMCID: PMC5890636
- DOI: 10.1136/annrheumdis-2017-211912
Patterns and predictors of skin score change in early diffuse systemic sclerosis from the European Scleroderma Observational Study
Abstract
Objectives: Our aim was to use the opportunity provided by the European Scleroderma Observational Study to (1) identify and describe those patients with early diffuse cutaneous systemic sclerosis (dcSSc) with progressive skin thickness, and (2) derive prediction models for progression over 12 months, to inform future randomised controlled trials (RCTs).
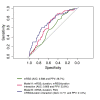
Methods: The modified Rodnan skin score (mRSS) was recorded every 3 months in 326 patients. 'Progressors' were defined as those experiencing a 5-unit and 25% increase in mRSS score over 12 months (±3 months). Logistic models were fitted to predict progression and, using receiver operating characteristic (ROC) curves, were compared on the basis of the area under curve (AUC), accuracy and positive predictive value (PPV).
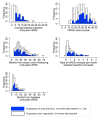
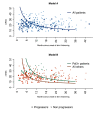
Results: 66 patients (22.5%) progressed, 227 (77.5%) did not (33 could not have their status assessed due to insufficient data). Progressors had shorter disease duration (median 8.1 vs 12.6 months, P=0.001) and lower mRSS (median 19 vs 21 units, P=0.030) than non-progressors. Skin score was highest, and peaked earliest, in the anti-RNA polymerase III (Pol3+) subgroup (n=50). A first predictive model (including mRSS, duration of skin thickening and their interaction) had an accuracy of 60.9%, AUC of 0.666 and PPV of 33.8%. By adding a variable for Pol3 positivity, the model reached an accuracy of 71%, AUC of 0.711 and PPV of 41%.
Conclusions: Two prediction models for progressive skin thickening were derived, for use both in clinical practice and for cohort enrichment in RCTs. These models will inform recruitment into the many clinical trials of dcSSc projected for the coming years.
Trial registration number: NCT02339441.
Keywords: autoantibodies; outcomes research; systemic sclerosis.
© Article author(s) (or their employer(s) unless otherwise stated in the text of the article) 2018. All rights reserved. No commercial use is permitted unless otherwise expressly granted.
Conflict of interest statement
Competing interests: ALH has done consultancy work for Actelion, served on a Data Safety Monitoring Board for Apricus, received research funding and speaker’s fees from Actelion, and speaker’s fees from GSK. JHWD has consultancy relationships and/or has received research funding from Actelion, BMS, Celgene, Bayer Pharma, Boehringer Ingelheim, JB Therapeutics, Sanofi-Aventis, Novartis, UCB, GSK, Array BioPharma, Active Biotech, Galapagos, Inventiva, Medac, Pfizer, Anamar and RuiYi, and is stock owner of 4D Science. OD has received consultancy fees from 4D Science, Actelion, Active Biotech, Bayer, Biogenidec, BMS, Boehringer Ingelheim, EpiPharm, Ergonex, espeRare Foundation, Genentech/Roche, GSK, Inventiva, Lilly, Medac, Medimmune, Pharmacyclics, Pfizer, Serodapharm, Sinoxa and UCB, and received research grants from Actelion, Bayer, Boehringer Ingelheim, Ergonex, Pfizer and Sanofi, and has a patent mir-29 for the treatment of systemic sclerosis licensed. WJG has received teaching fees from Pfizer. CA has served as a consultant for AbbVie, Pfizer, Roche, UCB, MSD, BMS and Novartis, and has received research funding and speaker fees from AbbVie, Pfizer, Roche, UCB, MSD, BMS and Novartis. FCH has received research funding from Actelion. MEA has undertaken advisory board work and received honoraria from Actelion, and received speaker’s fees from Bristol-Myers Squibb. NSD has done consultancy for AbbVie, Pfizer, Roche and MSD, and received speaker’s fees from AbbVie, Boehringer-Ingelheim, Pfizer, Richter Gedeon, Roche and MSD. HG has done consultancy work and received honoraria from Actelion. UM-L is funded in part by EUSTAR, EULAR and the European Community (Desscipher programme). JMvL has received honoraria from Eli Lilly, Pfizer, Roche, MSD and BMS. SP has received research grants from Actelion Pharmaceuticals Australia, Bayer, GlaxoSmithKline Australia and Pfizer, and speaker fees from Actelion. AR receives funding from AstraZeneca. CPD has done consultancy for GSK, Actelion, Bayer, Inventiva and Merck-Serono, received research grant funding from GSK, Actelion, CSL Behring and Inventiva, received speaker’s fees from Bayer and given trial advice to Merck-Serono.
Figures
References
-
- Clements P, Lachenbruch P, Siebold J, et al. . Inter and intraobserver variability of total skin thickness score (modified Rodnan TSS) in systemic sclerosis. J Rheumatol 1995;22:1281–5. - PubMed
-
- Steen VD, Medsger TA, Rodnan GP. D-Penicillamine therapy in progressive systemic sclerosis (scleroderma): a retrospective analysis. Ann Intern Med 1982;97:652–9. - PubMed
-
- Clements PJ, Furst DE, Wong WK, et al. . High-dose versus low-dose D-penicillamine in early diffuse systemic sclerosis: analysis of a two-year, double-blind, randomized, controlled clinical trial. Arthritis Rheum 1999;42:1194–203. 10.1002/1529-0131(199906)42:6<1194::AID-ANR16>3.0.CO;2-7 - DOI - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

