Targeting the Prostacyclin Pathway with Selexipag in Patients with Pulmonary Arterial Hypertension Receiving Double Combination Therapy: Insights from the Randomized Controlled GRIPHON Study
- PMID: 29307087
- PMCID: PMC5772136
- DOI: 10.1007/s40256-017-0262-z
Targeting the Prostacyclin Pathway with Selexipag in Patients with Pulmonary Arterial Hypertension Receiving Double Combination Therapy: Insights from the Randomized Controlled GRIPHON Study
Abstract
Background: In pulmonary arterial hypertension (PAH), combination therapy is an important treatment strategy. Although randomized controlled trial data are available to support the combination of two therapies, data regarding triple combination therapy are few.
Objective: The phase III GRIPHON trial enrolled 1156 patients with PAH, including 376 receiving background double combination therapy. We evaluated the efficacy and safety of selexipag as a third agent in these patients and further analyzed this subgroup according to symptom burden at baseline as indicated by World Health Organization (WHO) functional class (FC).
Methods: In this post hoc analysis, hazard ratios (HRs) and 95% confidence intervals (CI) were calculated using Cox proportional-hazard models to determine response to selexipag versus placebo on the composite primary endpoint of morbidity/mortality. Baseline characteristics and adverse events were summarized descriptively.
Results: Of 376 patients receiving background endothelin receptor antagonist (ERA) and phosphodiesterase-5 inhibitor (PDE-5i) therapy, 115 had WHO FC II symptoms and 255 had WHO FC III symptoms at baseline. The impact on the primary endpoint of adding selexipag versus placebo to double combination therapy was consistent with the effect in the overall population (HR 0.63; 95% CI 0.44-0.90) as well as in patients with WHO FC II and III symptoms. Compared with the overall population, discontinuations due to an adverse event were higher when selexipag was added to background double combination therapy; no safety concerns were identified.
Conclusion: The addition of selexipag to background double combination therapy with an ERA and PDE-5i provides an incremental benefit similar to that seen in the overall population, including in patients with WHO FC II or III symptoms at baseline. CLINICALTRIALS.
Gov identifier: NCT01106014.
Conflict of interest statement
Funding
This study was funded by Actelion Pharmaceuticals Ltd (Allschwil, Switzerland). Sally Dempster, Ph.D., and Ruth Lloyd, Ph.D. (nspm Ltd, Meggen, Switzerland) provided medical writing support funded by Actelion Pharmaceuticals Ltd.
Conflict of interest
Dr. Channick has served as a steering committee member for Actelion Pharmaceuticals Ltd, has served on an advisory board for Actelion Pharmaceuticals Ltd and Bayer, has received consultancy fees from Bayer, and has received research grants from Actelion Pharmaceuticals Ltd and United Therapeutics. Dr. Chin has served as a steering committee member for Actelion Pharmaceuticals Ltd; has received research grants from Actelion Pharmaceuticals Ltd, Bayer Healthcare, Gilead Sciences Inc., GeNO, NIH, United Therapeutics and SoniVie; and has received consultancy fees from Actelion Pharmaceuticals Ltd and United Therapeutics. Dr. Coghlan has received consultancy fees and honorarium from Actelion Pharmaceuticals Ltd and GlaxoSmithKline, honorarium from Bayer, and congress fees from MSD. Dr. Di Scala is a full-time employee of Actelion Pharmaceuticals Ltd. Professor Gaine has served as a steering committee member for and received research grants from Actelion Pharmaceuticals Ltd; has received speaker fees from Actelion Pharmaceuticals Ltd, Bayer, GlaxoSmithKline, MSD, and United Therapeutics; has received advisory board fees from Actelion Pharmaceuticals Ltd, Bayer, Novartis, Pfizer, and Daiichi-Sankyo; has received travel support from Actelion Pharmaceuticals Ltd, Bayer, GlaxoSmithKline, Novartis, and Daiichi-Sankyo; and has served on a data safety monitoring board for GlaxoSmithKline, Novartis, and United Therapeutics. Prof. Galiè has served as a steering committee member for Actelion Pharmaceuticals Ltd; has received research grants and consultancy fees from Actelion Pharmaceuticals Ltd, Bayer, GlaxoSmithKline, and Pfizer; and has received speaker fees from MSD. Prof. Ghofrani has served as a steering committee member for Actelion Pharmaceuticals Ltd; has received advisory board and speaker fees from Actelion Pharmaceuticals Ltd, Bayer, GlaxoSmithKline, Novartis, and Pfizer; has received consultancy fees from Actelion Pharmaceuticals Ltd, Bayer, Bellerophon Pulse technologies, GlaxoSmithKline, MSD, Novartis, and Pfizer; has received travel support from Actelion Pharmaceuticals Ltd, Bayer, GlaxoSmithKline, MSD, Novartis, and Pfizer; and has received research grants from Actelion Pharmaceuticals Ltd and Deutsche Forschungsgemeinschaft. Prof. Hoeper has served as a steering committee member for Actelion Pharmaceuticals Ltd; has received lecture and consultancy fees from Actelion Pharmaceuticals Ltd, Bayer, GlaxoSmithKline, MSD, and Pfizer; has received consultancy fees from Gilead; and has received research grants from Actelion Pharmaceuticals Ltd. Prof. Lang has served as a steering committee member for Actelion Pharmaceuticals Ltd; has received speaker fees from Actelion Pharmaceuticals Ltd, Bayer, and GlaxoSmithKline; and has received research grants from Actelion Pharmaceuticals Ltd, AOP Orphan Pharmaceuticals, and Bayer. Prof. McLaughlin has received consultancy fees from and served as an advisory board member for Actelion Pharmaceuticals Ltd, Arena Pharmaceuticals, Bayer, Gilead, Ikaria, and United therapeutics; has served as a steering committee member for Actelion Pharmaceuticals Ltd, Arena Pharmaceuticals, Bayer, and Gilead; has received personal fees from Steadymed Therapeutics and St Jude Medical; has received travel support from Actelion Pharmaceuticals Ltd, Arena Pharmaceuticals, Bayer, Gilead, Ikaria, and United Therapeutics; and has received research grants from Actelion Pharmaceuticals Ltd, Arena Pharmaceuticals, Bayer, Eiger, Gilead, Ikaria, Novartis, and SoniVie Ltd. Dr Preiss is a full-time employee of Actelion Pharmaceuticals Ltd. Prof. Rubin has received grant support from and served as a steering committee member for Actelion Pharmaceuticals Ltd; has received consultancy fees and travel support from Actelion Pharmaceuticals Ltd, Arena Pharmaceuticals, GeNO Pharmaceuticals, Gilead, Karos Pharmaceuticals, Pfizer, and SoniVie Ltd; and has three patents issued. Prof. Simonneau has served as a steering committee member for and received research grants from Actelion Pharmaceuticals Ltd and Bayer; has received speaker and consultancy fees from Actelion Pharmaceuticals Ltd, Bayer, GlaxoSmithKline, MSD, and Pfizer. Prof. Sitbon has served as a steering committee member for Actelion Pharmaceuticals Ltd; has served as an advisory board member for and received research grants from Actelion Pharmaceuticals Ltd, Bayer Healthcare, GlaxoSmithKline, and MSD; has received consultancy fees from Actelion Pharmaceuticals Ltd, Bayer Healthcare, GlaxoSmithKline, MSD, and United Therapeutics; has received speaker fees from Actelion Pharmaceuticals Ltd, Bayer Healthcare, GlaxoSmithKline, and United Therapeutics; and has received writing assistance from Actelion Pharmaceuticals Ltd and GlaxoSmithKline. Prof. Tapson has served as a steering committee member for Actelion Pharmaceuticals Ltd, Bayer, and United Therapeutics; has received research grants from Actelion Pharmaceuticals Ltd, Bayer, BIO2 Medical, Daiichi-Sankyo, Inari, Janssen, EKOS/BTG, Arena, Reata, Eiger, and United Therapeutics; has received consulting fees from Actelion Pharmaceuticals Ltd, Bayer, BiO2 Medical, United Therapeutics, Janssen, Arena, Reata, and Vwave medical; and has received lecture honorarium from Actelion, EKOS/BTG, Gilead Sciences, Bayer, and Janssen.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Figures
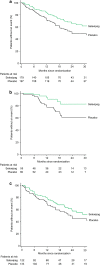

References
-
- Galiè N, Humbert M, Vachiery JL, et al. 2015 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension: The Joint Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS): endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC), International Society for Heart and Lung Transplantation (ISHLT) Eur Heart J. 2016;37:67–119. doi: 10.1093/eurheartj/ehv317. - DOI - PubMed
-
- Galiè N, Humbert M, Vachiery JL, et al. 2015 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension: The Joint Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS): endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC), International Society for Heart and Lung Transplantation (ISHLT) Eur Respir J. 2015;46:903–975. doi: 10.1183/13993003.01032-2015. - DOI - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

