Impact of Nicotine Exposure on Hair Cell Toxicity and Embryotoxicity During Zebrafish Development
- PMID: 29307133
- PMCID: PMC5951065
- DOI: 10.21053/ceo.2017.00857
Impact of Nicotine Exposure on Hair Cell Toxicity and Embryotoxicity During Zebrafish Development
Abstract
Objectives: Nicotine has various adverse effects including negative impacts associated with maternal exposure. In the current study, we examined nicotine-induced damage of hair cells and embryotoxicity during zebrafish development.
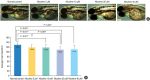
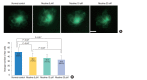
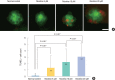
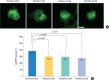
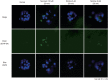
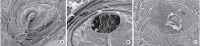
Methods: Zebrafish embryos were exposed to nicotine at several concentrations (5, 10, 20, and 40 μM) and embryotoxicity were evaluated at 72 hours, including hatching rate, mortality, teratogenicity rate, and heart rate. Hair cells within the supraorbital (SO1 and SO2), otic (O1), and occipital (OC1) neuromasts were identified at 120 hours. Apoptosis and mitochondrial damage of hair cells were analyzed using TUNEL (terminal deoxynucleotidyl transferase-mediated dUTP-biotin nick end labeling) and DASPEI (2-[4-(dimethylamino)styryl]-N-ethylpyridinium iodide) assays, respectively, and changes of ultrastructure were observed by scanning electron microscopy.
Results: The control group without nicotine appeared normal with overall mortality and teratogenicity rate <5%. The hatching rate and mortality rate was not significantly different according to nicotine concentration (n=400 each). The abnormal morphology rate (n=400) increased and heart rate (n=150) decreased with increasing nicotine concentration (P<0.05). Nicotine-induced hair cell damage significantly increased as nicotine concentration increased. A significantly greater number of TUNEL-positive cells (P<0.01) and markedly smaller DASPEI area (P<0.01) were shown as nicotine concentration increased.
Conclusion: The current results suggest that nicotine induces dose-dependent hair cell toxicity in embryos by promoting apoptosis and mitochondrial and structural damage.
Keywords: Embryotoxicity; Hair Cells; Nicotine; Ototoxicity; Tobacco; Zebrafish.
Conflict of interest statement
No potential conflict of interest relevant to this article was reported.
Figures

References
-
- Gray NJ. Nicotine yesterday, today, and tomorrow: a global review. Nicotine Tob Res. 2014 Feb;16(2):128–36. - PubMed
-
- Abbott LC, Winzer-Serhan UH. Smoking during pregnancy: lessons learned from epidemiological studies and experimental studies using animal models. Crit Rev Toxicol. 2012 Apr;42(4):279–303. - PubMed
-
- Parker B, Connaughton VP. Effects of nicotine on growth and development in larval zebrafish. Zebrafish. 2007 Spring;4(1):59–68. - PubMed
-
- Yoo MH, Rah YC, Choi J, Park S, Park HC, Oh KH, et al. Embryotoxicity and hair cell toxicity of silver nanoparticles in zebrafish embryos. Int J Pediatr Otorhinolaryngol. 2016 Apr;83:168–74. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

