Sex chromosome contributions to sex differences in multiple sclerosis susceptibility and progression
- PMID: 29307297
- PMCID: PMC5823689
- DOI: 10.1177/1352458517737394
Sex chromosome contributions to sex differences in multiple sclerosis susceptibility and progression
Abstract
Background: Why are women more susceptible to multiple sclerosis, but men have worse disability progression? Sex differences in disease may be due to sex hormones, sex chromosomes, or both.
Objective: Determine whether differences in sex chromosomes can contribute to sex differences in multiple sclerosis using experimental autoimmune encephalomyelitis.
Methods: Sex chromosome transgenic mice, which permit the study of sex chromosomes not confounded by differences in sex hormones, were used to examine an effect of sex chromosomes on autoimmunity and neurodegeneration, focusing on X chromosome genes.
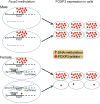
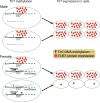
Results: T-lymphocyte DNA methylation studies of the X chromosome gene Foxp3 suggested that maternal versus paternal imprinting of X chromosome genes may underlie sex differences in autoimmunity. Bone marrow chimeras with the same immune system but different sex chromosomes in the central nervous system suggested that differential expression of the X chromosome gene Toll-like receptor 7 in neurons may contribute to sex differences in neurodegeneration.
Conclusion: Mapping the transcriptome and methylome in T lymphocytes and neurons in females versus males could reveal mechanisms underlying sex differences in autoimmunity and neurodegeneration.
Keywords: Multiple sclerosis; animal model; genetics; immunology; neurodegeneration; sex differences.
Conflict of interest statement
The Authors declare that there is no conflict of interest.
Figures
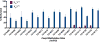

Similar articles
-
XY sex chromosome complement, compared with XX, in the CNS confers greater neurodegeneration during experimental autoimmune encephalomyelitis.Proc Natl Acad Sci U S A. 2014 Feb 18;111(7):2806-11. doi: 10.1073/pnas.1307091111. Epub 2014 Feb 3. Proc Natl Acad Sci U S A. 2014. PMID: 24550311 Free PMC article.
-
Sex- and species-specific contribution of CD99 to T cell costimulation during multiple sclerosis.Biol Sex Differ. 2024 May 15;15(1):41. doi: 10.1186/s13293-024-00618-y. Biol Sex Differ. 2024. PMID: 38750588 Free PMC article.
-
Male sex chromosomal complement exacerbates the pathogenicity of Th17 cells in a chronic model of central nervous system autoimmunity.Cell Rep. 2021 Mar 9;34(10):108833. doi: 10.1016/j.celrep.2021.108833. Cell Rep. 2021. PMID: 33691111
-
Sex differences regulate immune responses in experimental autoimmune encephalomyelitis and multiple sclerosis.Eur J Immunol. 2022 Jan;52(1):24-33. doi: 10.1002/eji.202149589. Epub 2021 Nov 13. Eur J Immunol. 2022. PMID: 34727577 Review.
-
Genomic imprinting: A missing piece of the Multiple Sclerosis puzzle?Int J Biochem Cell Biol. 2015 Oct;67:49-57. doi: 10.1016/j.biocel.2015.05.010. Epub 2015 May 19. Int J Biochem Cell Biol. 2015. PMID: 26002250 Review.
Cited by
-
The astrocyte transcriptome in EAE optic neuritis shows complement activation and reveals a sex difference in astrocytic C3 expression.Sci Rep. 2019 Jul 10;9(1):10010. doi: 10.1038/s41598-019-46232-6. Sci Rep. 2019. PMID: 31292459 Free PMC article.
-
Exercise-based telerehabilitation for patients with multiple sclerosis using physical activity: a systematic review.J Rehabil Med. 2024 Nov 13;56:jrm40641. doi: 10.2340/jrm.v56.40641. J Rehabil Med. 2024. PMID: 39539070 Free PMC article.
-
The X factor in neurodegeneration.J Exp Med. 2022 Dec 5;219(12):e20211488. doi: 10.1084/jem.20211488. Epub 2022 Nov 4. J Exp Med. 2022. PMID: 36331399 Free PMC article.
-
Sex effects across the lifespan in women with multiple sclerosis.Ther Adv Neurol Disord. 2020 Jul 1;13:1756286420936166. doi: 10.1177/1756286420936166. eCollection 2020. Ther Adv Neurol Disord. 2020. PMID: 32655689 Free PMC article. Review.
-
Forkhead Box P3 Methylation and Expression in Men with Obstructive Sleep Apnea.Int J Mol Sci. 2020 Mar 23;21(6):2233. doi: 10.3390/ijms21062233. Int J Mol Sci. 2020. PMID: 32210181 Free PMC article.
References
-
- Ontaneda D, Thompson AJ, Fox RJ, Cohen JA. Progressive multiple sclerosis: prospects for disease therapy, repair, and restoration of function. Lancet. 2017;389:1357–66. - PubMed
-
- Mahad DH, Trapp BD, Lassmann H. Pathological mechanisms in progressive multiple sclerosis. Lancet Neurol. 2015;14:183–93. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

