Brain metastases: neuroimaging
- PMID: 29307364
- PMCID: PMC6118134
- DOI: 10.1016/B978-0-12-811161-1.00007-4
Brain metastases: neuroimaging
Abstract
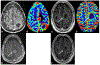
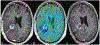
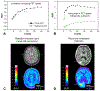
Magnetic resonance imaging (MRI) is the cornerstone for evaluating patients with brain masses such as primary and metastatic tumors. Important challenges in effectively detecting and diagnosing brain metastases and in accurately characterizing their subsequent response to treatment remain. These difficulties include discriminating metastases from potential mimics such as primary brain tumors and infection, detecting small metastases, and differentiating treatment response from tumor recurrence and progression. Optimal patient management could be benefited by improved and well-validated prognostic and predictive imaging markers, as well as early response markers to identify successful treatment prior to changes in tumor size. To address these fundamental needs, newer MRI techniques including diffusion and perfusion imaging, MR spectroscopy, and positron emission tomography (PET) tracers beyond traditionally used 18-fluorodeoxyglucose are the subject of extensive ongoing investigations, with several promising avenues of added value already identified. These newer techniques provide a wealth of physiologic and metabolic information that may supplement standard MR evaluation, by providing the ability to monitor and characterize cellularity, angiogenesis, perfusion, pH, hypoxia, metabolite concentrations, and other critical features of malignancy. This chapter reviews standard and advanced imaging of brain metastases provided by computed tomography, MRI, and amino acid PET, focusing on potential biomarkers that can serve as problem-solving tools in the clinical management of patients with brain metastases.
Keywords: MRI; PET; amino acid; brain; diffusion; imaging; metastasis; perfusion.
Copyright © 2018 Elsevier B.V. All rights reserved.
Figures



References
-
- Abdoli M, Chakraborty S, MacLean HJ et al. (2016). The evaluation of MRI diffusion values of active demyelinating lesions in multiple sclerosis. Mult Scler Relat Disord 10: 97–102. - PubMed
-
- Abe T, Mizobuchi Y, Sako W et al. (2015). Clinical significance of discrepancy between arterial spin labeling images and contrast-enhanced images in the diagnosis of brain tumors. Magn Reson Med Sci 14: 313–319. - PubMed
-
- Almeida-Freitas DB, Pinho MC, Otaduy MCG et al. (2014). Assessment of irradiated brain metastases using dynamic contrast-enhanced magnetic resonance imaging. Neuroradiology 56: 437–443. - PubMed
-
- Anzalone N, Essig M, Lee SK et al. (2013). Optimizing contrast-enhanced magnetic resonance imaging characterization of brain metastases: relevance to stereotactic radiosurgery. Neurosurgery 72: 691–701. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

