Prediction of cervical cancer incidence in England, UK, up to 2040, under four scenarios: a modelling study
- PMID: 29307386
- PMCID: PMC5765529
- DOI: 10.1016/S2468-2667(17)30222-0
Prediction of cervical cancer incidence in England, UK, up to 2040, under four scenarios: a modelling study
Abstract
Background: In the next 25 years, the epidemiology of cervical cancer in England, UK, will change: human papillomavirus (HPV) screening will be the primary test for cervical cancer. Additionally, the proportion of women screened regularly is decreasing and women who received the HPV vaccine are due to attend screening for the first time. Therefore, we aimed to estimate how vaccination against HPV, changes to the screening test, and falling screening coverage will affect cervical cancer incidence in England up to 2040.
Methods: We did a data modelling study that combined results from population modelling of incidence trends, observable data from the individual level with use of a generalised linear model, and microsimulation of unobservable disease states. We estimated age-specific absolute risks of cervical cancer in the absence of screening (derived from individual level data). We used an age period cohort model to estimate birth cohort effects. We multiplied the absolute risks by the age cohort effects to provide absolute risks of cervical cancer for unscreened women in different birth cohorts. We obtained relative risks (RRs) of cervical cancer by screening history (never screened, regularly screened, or lapsed attender) using data from a population-based case-control study for unvaccinated women, and using a microsimulation model for vaccinated women. RRs of primary HPV screening were relative to cytology. We used the proportion of women in each 5-year age group (25-29 years to 75-79 years) and 5-year period (2016-20 to 2036-40) who have a combination of screening and vaccination history, and weighted to estimate the population incidence. The primary outcome was the number of cases and rates per 100 000 women under four scenarios: no changes to current screening coverage or vaccine uptake and HPV primary testing from 2019 (status quo), changing the year in which HPV primary testing is introduced, introduction of the nine-valent vaccine, and changes to cervical screening coverage.
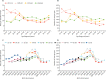
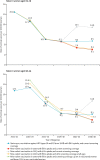
Findings: The status quo scenario estimated that the peak age of cancer diagnosis will shift from the ages of 25-29 years in 2011-15 to 55-59 years in 2036-40. Unvaccinated women born between 1975 and 1990 were predicted to have a relatively high risk of cervical cancer throughout their lives. Introduction of primary HPV screening from 2019 could reduce age-standardised rates of cervical cancer at ages 25-64 years by 19%, from 15·1 in 2016 to 12·2 per 100 000 women as soon as 2028. Vaccination against HPV types 16 and 18 (HPV 16/18) could see cervical cancer rates in women aged 25-29 years decrease by 55% (from 20·9 in 2011-15 to 9·5 per 100 000 women by 2036-40), and introduction of nine-valent vaccination from 2019 compared with continuing vaccination against HPV 16/18 will reduce rates by a further 36% (from 9·5 to 6·1 per 100 000 women) by 2036-40. Women born before 1991 will not benefit directly from vaccination; therefore, despite vaccination and primary HPV screening with current screening coverage, European age-standardised rates of cervical cancer at ages 25-79 years will decrease by only 10% (from 12·8 in 2011-15 to 11·5 per 100 000 women in 2036-40). If screening coverage fell to 50%, European age-standardised rates could increase by 27% (from 12·8 to 16·3 per 100 000 by 2036-40).
Interpretation: Going forward, focus should be placed on scenarios that offer less intensive screening for vaccinated women and more on increasing coverage and incorporation of new technologies to enhance current cervical screening among unvaccinated women.
Funding: Jo's Cervical Cancer Trust and Cancer Research UK.
Copyright © 2018 The Author(s). Published by Elsevier Ltd. This is an Open Access article under the CC BY 4.0 license. Published by Elsevier Ltd.. All rights reserved.
Figures

Comment in
-
Accelerating cervical cancer control and prevention.Lancet Public Health. 2018 Jan;3(1):e6-e7. doi: 10.1016/S2468-2667(17)30242-6. Epub 2017 Dec 19. Lancet Public Health. 2018. PMID: 29307389 No abstract available.
Similar articles
-
The clinical effectiveness and cost-effectiveness of primary human papillomavirus cervical screening in England: extended follow-up of the ARTISTIC randomised trial cohort through three screening rounds.Health Technol Assess. 2014 Apr;18(23):1-196. doi: 10.3310/hta18230. Health Technol Assess. 2014. PMID: 24762804 Free PMC article. Clinical Trial.
-
The projected timeframe until cervical cancer elimination in Australia: a modelling study.Lancet Public Health. 2019 Jan;4(1):e19-e27. doi: 10.1016/S2468-2667(18)30183-X. Epub 2018 Oct 2. Lancet Public Health. 2019. PMID: 30291040
-
Impact of scaled up human papillomavirus vaccination and cervical screening and the potential for global elimination of cervical cancer in 181 countries, 2020-99: a modelling study.Lancet Oncol. 2019 Mar;20(3):394-407. doi: 10.1016/S1470-2045(18)30836-2. Epub 2019 Feb 19. Lancet Oncol. 2019. PMID: 30795950
-
Prophylaxis of cervical cancer and related cervical disease: a review of the cost-effectiveness of vaccination against oncogenic HPV types.J Manag Care Pharm. 2010 Apr;16(3):217-30. doi: 10.18553/jmcp.2010.16.3.217. J Manag Care Pharm. 2010. PMID: 20331326 Free PMC article. Review.
-
What's next? Perspectives and future needs of cervical screening in Europe in the era of molecular testing and vaccination.Eur J Cancer. 2009 Oct;45(15):2714-21. doi: 10.1016/j.ejca.2009.07.024. Epub 2009 Aug 18. Eur J Cancer. 2009. PMID: 19695870 Review.
Cited by
-
Factors associated with cervical screening coverage: a longitudinal analysis of English general practices from 2013 to 2022.J Public Health (Oxf). 2024 Feb 23;46(1):e43-e50. doi: 10.1093/pubmed/fdad275. J Public Health (Oxf). 2024. PMID: 38148290 Free PMC article.
-
25 year trends in cancer incidence and mortality among adults aged 35-69 years in the UK, 1993-2018: retrospective secondary analysis.BMJ. 2024 Mar 13;384:e076962. doi: 10.1136/bmj-2023-076962. BMJ. 2024. PMID: 38479774 Free PMC article.
-
The Association of Molecular Biomarkers in the Diagnosis of Cervical Pre-Cancer and Cancer and Risk Factors in Senegalese.Asian Pac J Cancer Prev. 2020 Nov 1;21(11):3221-3227. doi: 10.31557/APJCP.2020.21.11.3221. Asian Pac J Cancer Prev. 2020. PMID: 33247678 Free PMC article.
-
Interventions targeted at women to encourage the uptake of cervical screening.Cochrane Database Syst Rev. 2021 Sep 6;9(9):CD002834. doi: 10.1002/14651858.CD002834.pub3. Cochrane Database Syst Rev. 2021. PMID: 34694000 Free PMC article.
-
Management of cervical cancer in pregnancy in a low resource setting: a rare case report.BMC Pregnancy Childbirth. 2024 Sep 12;24(1):597. doi: 10.1186/s12884-024-06716-4. BMC Pregnancy Childbirth. 2024. PMID: 39266963 Free PMC article.
References
-
- Public Health England HPV primary screening in the cervical screening programme. 2016. https://phescreening.blog.gov.uk/2016/04/13/hpv-primary-screening-in-the... (accessed June 15, 2016).
-
- Sanofi Pasteur MSD. GARDASIL 9: 2-dose schedule approved in Europe. 2016. http://www.multivu.com/players/uk/7805051-gardasil-9-2-dose-approved-in-... (accessed Sept 7, 2016).
-
- Office for National Statistics National population projections: 2012-based statistical bulletin. 2013. http://www.ons.gov.uk/peoplepopulationandcommunity/populationandmigratio... (accessed Sept 1, 2017).
-
- Public Health England Audit of invasive cervical cancers. 2006. https://www.gov.uk/government/publications/cervical-screening-auditing-p... (accessed Nov 2, 2015).
-
- Office for National Statistics Cancer registration statistics, England statistical bulletins. http://www.ons.gov.uk/peoplepopulationandcommunity/healthandsocialcare/c... (accessed Sept 1, 2017).
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

