Nucleus Accumbens Subnuclei Regulate Motivated Behavior via Direct Inhibition and Disinhibition of VTA Dopamine Subpopulations
- PMID: 29307710
- PMCID: PMC5773387
- DOI: 10.1016/j.neuron.2017.12.022
Nucleus Accumbens Subnuclei Regulate Motivated Behavior via Direct Inhibition and Disinhibition of VTA Dopamine Subpopulations
Abstract
Mesolimbic dopamine (DA) neurons play a central role in motivation and reward processing. Although the activity of these mesolimbic DA neurons is controlled by afferent inputs, little is known about the circuits in which they are embedded. Using retrograde tracing, electrophysiology, optogenetics, and behavioral assays, we identify principles of afferent-specific control in the mesolimbic DA system. Neurons in the medial shell subdivision of the nucleus accumbens (NAc) exert direct inhibitory control over two separate populations of mesolimbic DA neurons by activating different GABA receptor subtypes. In contrast, NAc lateral shell neurons mainly synapse onto ventral tegmental area (VTA) GABA neurons, resulting in disinhibition of DA neurons that project back to the NAc lateral shell. Lastly, we establish a critical role for NAc subregion-specific input to the VTA underlying motivated behavior. Collectively, our results suggest a distinction in the incorporation of inhibitory inputs between different subtypes of mesolimbic DA neurons.
Keywords: GABA; dopamine; mesolimbic; motivation; nucleus accumbens; reward; ventral tegmental area.
Copyright © 2017 Elsevier Inc. All rights reserved.
Conflict of interest statement
The authors declare no competing interests.
Figures




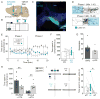
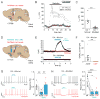


Comment in
-
Spiraling Connectivity of NAc-VTA Circuitry.Neuron. 2018 Jan 17;97(2):261-262. doi: 10.1016/j.neuron.2017.12.046. Neuron. 2018. PMID: 29346748
References
-
- Bjorklund A, Dunnett SB. Dopamine neuron systems in the brain: an update. Trends Neurosci. 2007;30:194–202. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

