Microbial modulation of cardiovascular disease
- PMID: 29307889
- PMCID: PMC5885760
- DOI: 10.1038/nrmicro.2017.149
Microbial modulation of cardiovascular disease
Abstract
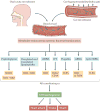
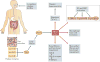
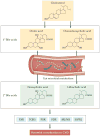
Although diet has long been known to contribute to the pathogenesis of cardiovascular disease (CVD), research over the past decade has revealed an unexpected interplay between nutrient intake, gut microbial metabolism and the host to modify the risk of developing CVD. Microbial-associated molecular patterns are sensed by host pattern recognition receptors and have been suggested to drive CVD pathogenesis. In addition, the host microbiota produces various metabolites, such as trimethylamine-N-oxide, short-chain fatty acids and secondary bile acids, that affect CVD pathogenesis. These recent advances support the notion that targeting the interactions between the host and microorganisms may hold promise for the prevention or treatment of CVD. In this Review, we summarize our current knowledge of the gut microbial mechanisms that drive CVD, with special emphasis on therapeutic interventions, and we highlight the need to establish causal links between microbial pathways and CVD pathogenesis.
Conflict of interest statement
S.L.H. is named as inventor on pending and issued patents held by the Cleveland Clinic relating to cardiovascular diagnostics and therapeutics. He is also a paid consultant for Esperion and P&G, and has received research funds from Astra Zeneca, P&G, Pfizer Inc., Roche Diagnostics, and Takeda. S.L.H. has also received royalty payments for inventions or discoveries related to cardiovascular diagnostics or therapeutics from Cleveland HeartLab, Esperion and Siemens.
J. M. B declares no competing interests.
Figures
References
-
- Mozaffarian D, et al. Executive summary: heart disease and stroke statistics - 2016 update: a report from the American Heart Assocation. Circulation. 2016;133:447–454. - PubMed
-
- Ardissino D, et al. Influence of 9p21.3 genetic variants on clinical and angiographic outcomes in early-onset myocardial infarction. J. Am. Coll. Cardiol. 2011;58:426–34. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

