Genomic medicine for kidney disease
- PMID: 29307893
- PMCID: PMC5997488
- DOI: 10.1038/nrneph.2017.167
Genomic medicine for kidney disease
Abstract
Technologies such as next-generation sequencing and chromosomal microarray have advanced the understanding of the molecular pathogenesis of a variety of renal disorders. Genetic findings are increasingly used to inform the clinical management of many nephropathies, enabling targeted disease surveillance, choice of therapy, and family counselling. Genetic analysis has excellent diagnostic utility in paediatric nephrology, as illustrated by sequencing studies of patients with congenital anomalies of the kidney and urinary tract and steroid-resistant nephrotic syndrome. Although additional investigation is needed, pilot studies suggest that genetic testing can also provide similar diagnostic insight among adult patients. Reaching a genetic diagnosis first involves choosing the appropriate testing modality, as guided by the clinical presentation of the patient and the number of potential genes associated with the suspected nephropathy. Genome-wide sequencing increases diagnostic sensitivity relative to targeted panels, but holds the challenges of identifying causal variants in the vast amount of data generated and interpreting secondary findings. In order to realize the promise of genomic medicine for kidney disease, many technical, logistical, and ethical questions that accompany the implementation of genetic testing in nephrology must be addressed. The creation of evidence-based guidelines for the utilization and implementation of genetic testing in nephrology will help to translate genetic knowledge into improved clinical outcomes for patients with kidney disease.
Conflict of interest statement
The authors declare no competing interests.
Figures
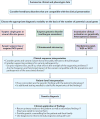
References
-
- Devuyst O, et al. Rare inherited kidney diseases: challenges, opportunities, and perspectives. Lancet. 2014;383:1844–1859. This review provides a comprehensive overview of the major Mendelian forms of CKD and examines the existing challenges to and future opportunities for effective clinical detection and management. - PMC - PubMed
-
- Jha V, et al. Chronic kidney disease: global dimension and perspectives. Lancet. 2013;382:260–272. - PubMed
-
- Wuhl E, et al. Renal replacement therapy for rare diseases affecting the kidney: an analysis of the ERA-EDTA Registry. Nephrol. Dial. Transplant. 2014;29(Suppl. 4):iv1–iv8. - PubMed
-
- Ingelfinger JR, Kalantar-Zadeh K, Schaefer F World Kidney Day Steering Committee. World Kidney Day 2016: averting the legacy of kidney disease — focus on childhood. Pediatr. Nephrol. 2016;31:343–348. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

