Mesoporous inorganic salts with crystal defects: unusual catalysts and catalyst supports
- PMID: 29308132
- PMCID: PMC5639790
- DOI: 10.1039/c4sc03736g
Mesoporous inorganic salts with crystal defects: unusual catalysts and catalyst supports
Abstract
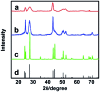
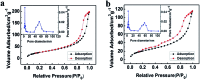
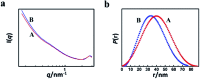
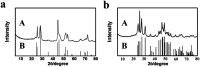
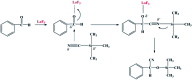
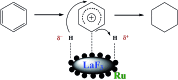
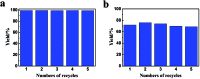
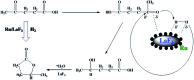
We proposed a strategy to synthesize mesoporous inorganic salt particles using the special properties of ionic liquid (IL) mixtures, and hollow mesoporous LaF3, NdF3, and YF3 particles were synthesized and characterized using different techniques. The size of the mesopores in the salt particles was about 4 nm, and the materials were full of crystal defects. The LaF3, NdF3 and YF3 particles were used as the catalysts for the cyanosilylation reaction of benzaldehyde using trimethylsilyl cyanide, and Ru/LaF3 and Ru/NdF3, in which Ru nanocatalysts were supported on the LaF3 and NdF3 particles with mesopores, were used to catalyze hydrogenations of benzene to cyclohexane and levulinic acid (LA) to γ-valerolactone (GVL). It was discovered that the activities of these catalysts were unprecedentedly high for these reactions. Detailed study showed that both the crystal defects and the mesopores in the salt particles played crucial roles for the extremely high catalytic activity.
Figures




Similar articles
-
Stable yolk-structured catalysts towards aqueous levulinic acid hydrogenation within a single Ru nanoparticle anchored inside the mesoporous shell of hollow carbon spheres.J Colloid Interface Sci. 2020 Sep 15;576:394-403. doi: 10.1016/j.jcis.2020.05.039. Epub 2020 May 16. J Colloid Interface Sci. 2020. PMID: 32460100
-
Hydrodeoxygenation of Levulinic Acid to γ-Valerolactone over Mesoporous Silica-Supported Cu-Ni Composite Catalysts.Molecules. 2022 Aug 24;27(17):5383. doi: 10.3390/molecules27175383. Molecules. 2022. PMID: 36080151 Free PMC article.
-
Functionalized Biochars as Supports for Ru/C Catalysts: Tunable and Efficient Materials for γ-Valerolactone Production.Nanomaterials (Basel). 2023 Mar 22;13(6):1129. doi: 10.3390/nano13061129. Nanomaterials (Basel). 2023. PMID: 36986022 Free PMC article.
-
Recent Advances in Ruthenium-Catalyzed Hydrogenation Reactions of Renewable Biomass-Derived Levulinic Acid in Aqueous Media.Front Chem. 2020 Apr 21;8:221. doi: 10.3389/fchem.2020.00221. eCollection 2020. Front Chem. 2020. PMID: 32373576 Free PMC article. Review.
-
Effects of Solid Acid Supports on the Bifunctional Catalysis of Levulinic Acid to γ-Valerolactone: Catalytic Activity and Stability.Chem Asian J. 2020 Apr 17;15(8):1182-1201. doi: 10.1002/asia.202000006. Epub 2020 Mar 10. Chem Asian J. 2020. PMID: 32012471 Review.
Cited by
-
Cyanosilylation of Aromatic Aldehydes by Cationic Ruthenium(II) Complexes of Benzimidazole-Derived O-Functionalized N-Heterocyclic Carbenes at Ambient Temperature under Solvent-Free Conditions.ACS Omega. 2018 Feb 14;3(2):1922-1938. doi: 10.1021/acsomega.7b02090. eCollection 2018 Feb 28. ACS Omega. 2018. PMID: 31458504 Free PMC article.
-
Deep eutectic solvent-assisted fabrication of zirconium phytate thin nanosheets for important biomass transformations.iScience. 2022 Aug 30;25(10):105039. doi: 10.1016/j.isci.2022.105039. eCollection 2022 Oct 21. iScience. 2022. PMID: 36147961 Free PMC article.
-
Heterogeneous conversion of CO2 into cyclic carbonates at ambient pressure catalyzed by ionothermal-derived meso-macroporous hierarchical poly(ionic liquid)s.Chem Sci. 2015 Dec 1;6(12):6916-6924. doi: 10.1039/c5sc02050f. Epub 2015 Aug 27. Chem Sci. 2015. PMID: 29861930 Free PMC article.
-
Carbon dioxide electroreduction to C2 products over copper-cuprous oxide derived from electrosynthesized copper complex.Nat Commun. 2019 Aug 26;10(1):3851. doi: 10.1038/s41467-019-11599-7. Nat Commun. 2019. PMID: 31451700 Free PMC article.
References
-
- Tozawa T., Jones J. T. A., Swamy S. I., Jiang S., Adams D. J., Shakespeare S., Clowes R., Bradshaw D., Hasell T., Chong S. Y., Tang C., Thompson S., Parker J., Trewin A., Bacsa J., Slawin A. M. Z., Steiner A., Cooper A. I. Nat. Mater. 2009;8:973. - PubMed
- Colson J. W., Woll A. R., Mukherjee A., Levendorf M. P., Spitler E. L., Shields V. B., Spencer M. G., Park J., Dichtel W. R. Science. 2011;332:228. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

