Modulation of inherent dynamical tendencies of the bisabolyl cation via preorganization in epi-isozizaene synthase
- PMID: 29308148
- PMCID: PMC5645776
- DOI: 10.1039/c4sc03782k
Modulation of inherent dynamical tendencies of the bisabolyl cation via preorganization in epi-isozizaene synthase
Abstract
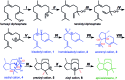
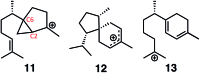
The relative importance of preorganization, selective transition state stabilization and inherent reactivity are assessed through quantum chemical and docking calculations for a sesquiterpene synthase (epi-isozizaene synthase, EIZS). Inherent reactivity of the bisabolyl cation, both static and dynamic, appears to determine the pathway to product, although preorganization and selective binding of the final transition state structure in the multi-step carbocation cascade that forms epi-isozizaene appear to play important roles.
Figures

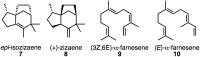

Similar articles
-
Structure-Based Engineering of a Sesquiterpene Cyclase to Generate an Alcohol Product: Conversion of epi-Isozizaene Synthase into α-Bisabolol Synthase.Biochemistry. 2024 Mar 19;63(6):797-805. doi: 10.1021/acs.biochem.3c00681. Epub 2024 Feb 29. Biochemistry. 2024. PMID: 38420671 Free PMC article.
-
An Aromatic Cluster in the Active Site of epi-Isozizaene Synthase Is an Electrostatic Toggle for Divergent Terpene Cyclization Pathways.Biochemistry. 2020 Dec 22;59(50):4744-4754. doi: 10.1021/acs.biochem.0c00876. Epub 2020 Dec 3. Biochemistry. 2020. PMID: 33270439 Free PMC article.
-
Consequences of conformational preorganization in sesquiterpene biosynthesis: theoretical studies on the formation of the bisabolene, curcumene, acoradiene, zizaene, cedrene, duprezianene, and sesquithuriferol sesquiterpenes.J Am Chem Soc. 2009 Jun 17;131(23):7999-8015. doi: 10.1021/ja9005332. J Am Chem Soc. 2009. PMID: 19469543
-
Importance of Inherent Substrate Reactivity in Enzyme-Promoted Carbocation Cyclization/Rearrangements.Angew Chem Int Ed Engl. 2017 Aug 14;56(34):10040-10045. doi: 10.1002/anie.201702363. Epub 2017 Jun 12. Angew Chem Int Ed Engl. 2017. PMID: 28349600 Review.
-
Preorganization and protein dynamics in enzyme catalysis.Chem Rec. 2002;2(1):24-36. doi: 10.1002/tcr.10009. Chem Rec. 2002. PMID: 11933259 Review.
Cited by
-
Dynamic behavior of rearranging carbocations - implications for terpene biosynthesis.Beilstein J Org Chem. 2016 Feb 29;12:377-90. doi: 10.3762/bjoc.12.41. eCollection 2016. Beilstein J Org Chem. 2016. PMID: 27340434 Free PMC article. Review.
-
Stereochemical investigations on the biosynthesis of achiral (Z)-γ-bisabolene in Cryptosporangium arvum.Beilstein J Org Chem. 2019 Mar 27;15:789-794. doi: 10.3762/bjoc.15.75. eCollection 2019. Beilstein J Org Chem. 2019. PMID: 30992727 Free PMC article.
-
Carbocations and the Complex Flavor and Bouquet of Wine: Mechanistic Aspects of Terpene Biosynthesis in Wine Grapes.Molecules. 2015 Jun 11;20(6):10781-92. doi: 10.3390/molecules200610781. Molecules. 2015. PMID: 26111168 Free PMC article. Review.
-
Mechanistically informed predictions of binding modes for carbocation intermediates of a sesquiterpene synthase reaction.Chem Sci. 2016 Jul 1;7(7):4009-4015. doi: 10.1039/c6sc00635c. Epub 2016 Mar 21. Chem Sci. 2016. PMID: 30155043 Free PMC article.
-
Biosynthetic insights provided by unusual sesterterpenes from the medicinal herb Aletris farinosa.Chem Sci. 2015 Oct 1;6(10):5740-5745. doi: 10.1039/c5sc02056e. Epub 2015 Jul 6. Chem Sci. 2015. PMID: 29081941 Free PMC article.
References
-
- Stork G., Burgstahler A. W. J. Am. Chem. Soc. 1955;77:5068–5077.
-
- Eschenmoser A., Ruzicka L., Jeger O., Arigoni D. Helv. Chim. Acta. 1955;38:1890–1904.
-
- Hong Y. J., Tantillo D. J. J. Am. Chem. Soc. 2009;131:7999–8015. - PubMed
-
- Hong Y. J., Tantillo D. J. J. Am. Chem. Soc. 2014;136:2450–2463. - PubMed
-
- Hong Y. J., Tantillo D. J. J. Am. Chem. Soc. 2011;133:18249–18256. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

