Modulation of inherent dynamical tendencies of the bisabolyl cation via preorganization in epi-isozizaene synthase
- PMID: 29308148
- PMCID: PMC5645776
- DOI: 10.1039/c4sc03782k
Modulation of inherent dynamical tendencies of the bisabolyl cation via preorganization in epi-isozizaene synthase
Abstract
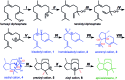
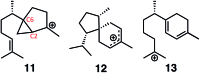
The relative importance of preorganization, selective transition state stabilization and inherent reactivity are assessed through quantum chemical and docking calculations for a sesquiterpene synthase (epi-isozizaene synthase, EIZS). Inherent reactivity of the bisabolyl cation, both static and dynamic, appears to determine the pathway to product, although preorganization and selective binding of the final transition state structure in the multi-step carbocation cascade that forms epi-isozizaene appear to play important roles.
Figures

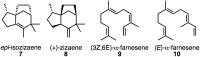

References
-
- Stork G., Burgstahler A. W. J. Am. Chem. Soc. 1955;77:5068–5077.
-
- Eschenmoser A., Ruzicka L., Jeger O., Arigoni D. Helv. Chim. Acta. 1955;38:1890–1904.
-
- Hong Y. J., Tantillo D. J. J. Am. Chem. Soc. 2009;131:7999–8015. - PubMed
-
- Hong Y. J., Tantillo D. J. J. Am. Chem. Soc. 2014;136:2450–2463. - PubMed
-
- Hong Y. J., Tantillo D. J. J. Am. Chem. Soc. 2011;133:18249–18256. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

