Systemic Inflammation, Coagulation, and Clinical Risk in the START Trial
- PMID: 29308409
- PMCID: PMC5751061
- DOI: 10.1093/ofid/ofx262
Systemic Inflammation, Coagulation, and Clinical Risk in the START Trial
Abstract
Background: The Strategic Timing of AntiRetroviral Treatment (START) trial demonstrated that immediate (at CD4+ >500 cells/µL) vs deferred (to CD4+ <350 cells/µL or AIDS) antiretroviral therapy (ART) initiation reduced risk for AIDS and serious non-AIDS (SNA). We investigated associations of inflammation, coagulation, and vascular injury biomarkers with AIDS, SNA or death, and the effect of immediate ART initiation.
Methods: Biomarkers were measured from stored plasma prior to randomization and at month 8. Associations of baseline biomarkers with event risk were estimated with Cox regression, pooled across groups, adjusted for age, gender, and treatment group, and stratified by region. Mean changes over 8 months were estimated and compared between the immediate and deferred ART arms using analysis of covariance models, adjusted for levels at entry.
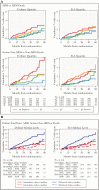
Results: Baseline biomarker levels were available for 4299 START participants (92%). Mean follow-up was 3.2 years. Higher levels of IL-6 and D-dimer were the only biomarkers associated with risk for AIDS, SNA or death, as well as the individual components of SNA and AIDS events (HRs ranged 1.37-1.41 per 2-fold higher level), even after adjustment for baseline CD4+ count, HIV RNA level, and other biomarkers. At month 8, biomarker levels were lower in the immediate arm by 12%-21%.
Conclusions: These data, combined with evidence from prior biomarker studies, demonstrate that IL-6 and D-dimer consistently predict clinical risk across a broad spectrum of CD4 counts for those both ART-naïve and treated. Research is needed to identify disease-modifying treatments that target inflammation beyond the effects of ART.
Keywords: HIV disease; coagulation; comorbidities; end-organ disease; inflammation risk.
Figures



References
-
- Mocroft A, Reiss P, Gasiorowski J et al. ; EuroSIDA Study Group. Serious fatal and nonfatal non-AIDS-defining illnesses in Europe. J Acquir Immune Defic Syndr 2010; 55:262–70. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

