Charge separation and carrier dynamics in donor-acceptor heterojunction photovoltaic systems
- PMID: 29308415
- PMCID: PMC5736396
- DOI: 10.1063/1.4996409
Charge separation and carrier dynamics in donor-acceptor heterojunction photovoltaic systems
Abstract
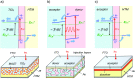
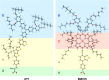
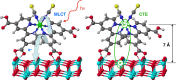
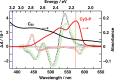
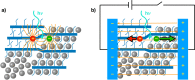
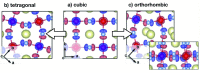
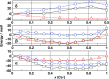
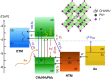
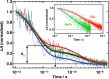
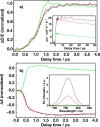
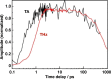
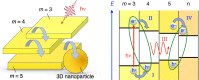
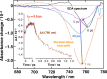
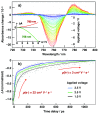
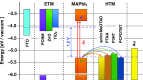
Electron transfer and subsequent charge separation across donor-acceptor heterojunctions remain the most important areas of study in the field of third-generation photovoltaics. In this context, it is particularly important to unravel the dynamics of individual ultrafast processes (such as photoinduced electron transfer, carrier trapping and association, and energy transfer and relaxation), which prevail in materials and at their interfaces. In the frame of the National Center of Competence in Research "Molecular Ultrafast Science and Technology," a research instrument of the Swiss National Science Foundation, several groups active in the field of ultrafast science in Switzerland have applied a number of complementary experimental techniques and computational simulation tools to scrutinize these critical photophysical phenomena. Structural, electronic, and transport properties of the materials and the detailed mechanisms of photoinduced charge separation in dye-sensitized solar cells, conjugated polymer- and small molecule-based organic photovoltaics, and high-efficiency lead halide perovskite solar energy converters have been scrutinized. Results yielded more than thirty research articles, an overview of which is provided here.
Figures

References
-
- Nazeeruddin M. K., Baranoff E., and Grätzel M., Sol. Energy 85, 1172 (2011).10.1016/j.solener.2011.01.018 - DOI
-
- Grätzel M. and Moser J.-E., in Electron Transfer in Chemistry, edited by Balzani V. and Gould I. R. ( Wiley-VCH, Weinheim, 2001), Vol. 5, p. 589.
-
- Moser J.-E., in Dye-Sensitized Solar Cells, edited by Kalyanasundaram K. ( EPFL Press, Lausanne, 2010), Chap. 11, p. 403.
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

