Anti-vascular endothelial growth factor (VEGF) drugs for treatment of retinopathy of prematurity
- PMID: 29308602
- PMCID: PMC6491066
- DOI: 10.1002/14651858.CD009734.pub3
Anti-vascular endothelial growth factor (VEGF) drugs for treatment of retinopathy of prematurity
Abstract
Background: Vascular endothelial growth factor (VEGF) plays a key role in angiogenesis in foetal life. Researchers have recently attempted to use anti-VEGF agents for the treatment of retinopathy of prematurity (ROP), a vasoproliferative disorder. The safety and efficacy of these agents in preterm infants with ROP is currently uncertain.
Objectives: To evaluate the efficacy and safety of anti-VEGF drugs when used either as monotherapy, that is without concomitant cryotherapy or laser therapy, or in combination with planned cryo/laser therapy in preterm infants with type 1 ROP (defined as zone I any stage with plus disease, zone I stage 3 with or without plus disease, or zone II stage 2 or 3 with plus disease).
Search methods: We searched the Cochrane Central Register of Controlled Trials (CENTRAL 2016, Issue 11), MEDLINE (1966 to 11 December 2016), Embase (1980 to 11 December 2016), CINAHL (1982 to 11 December 2016), and conference proceedings.
Selection criteria: Randomised or quasi-randomised controlled trials that evaluated the efficacy or safety of administration, or both, of anti-VEGF agents compared with conventional therapy in preterm infants with ROP.
Data collection and analysis: We used standard Cochrane and Cochrane Neonatal methods for data collection and analysis. We used the GRADE approach to assess the quality of the evidence.
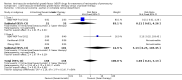
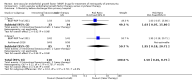
Main results: Six trials involving a total of 383 infants fulfilled the inclusion criteria. Five trials compared intravitreal bevacizumab (n = 4) or ranibizumab (n = 1) with conventional laser therapy (monotherapy), while the sixth study compared intravitreal pegaptanib plus conventional laser therapy with laser/cryotherapy (combination therapy).When used as monotherapy, bevacizumab/ranibizumab did not reduce the risk of complete or partial retinal detachment (3 studies; 272 infants; risk ratio (RR) 1.04, 95% confidence interval (CI) 0.21 to 5.13; risk difference (RD) 0.00, 95% CI -0.04 to 0.04; very low-quality evidence), mortality before discharge (2 studies; 229 infants; RR 1.50, 95% CI 0.26 to 8.75), corneal opacity requiring corneal transplant (1 study; 286 eyes; RR 0.34, 95% CI 0.01 to 8.26), or lens opacity requiring cataract removal (3 studies; 544 eyes; RR 0.15, 95% CI 0.01 to 2.79). The risk of recurrence of ROP requiring retreatment also did not differ between groups (2 studies; 193 infants; RR 0.88, 95% CI 0.47 to 1.63; RD -0.02, 95% CI -0.12 to 0.07; very low-quality evidence). Subgroup analysis showed a significant reduction in the risk of recurrence in infants with zone I ROP (RR 0.15, 95% CI 0.04 to 0.62), but an increased risk of recurrence in infants with zone II ROP (RR 2.53, 95% CI 1.01 to 6.32). Pooled analysis of studies that reported eye-level outcomes also revealed significant increase in the risk of recurrence of ROP in the eyes that received bevacizumab (RR 5.36, 95% CI 1.22 to 23.50; RD 0.10, 95% CI 0.03 to 0.17). Infants who received intravitreal bevacizumab had a significantly lower risk of refractive errors (very high myopia) at 30 months of age (1 study; 211 eyes; RR 0.06, 95% CI 0.02 to 0.20; RD -0.40, 95% CI -0.50 to -0.30; low-quality evidence).When used in combination with laser therapy, intravitreal pegaptanib was found to reduce the risk of retinal detachment when compared to laser/cryotherapy alone (152 eyes; RR 0.26, 95% CI 0.12 to 0.55; RD -0.29, 95% CI -0.42 to -0.16; low-quality evidence). The incidence of recurrence of ROP by 55 weeks' postmenstrual age was also lower in the pegaptanib + laser therapy group (76 infants; RR 0.29, 95% CI 0.12 to 0.7; RD -0.35, 95% CI -0.55 to -0.16; low-quality evidence). There was no difference in the risk of perioperative retinal haemorrhages between the two groups (152 eyes; RR 0.62, 95% CI 0.24 to 1.56; RD -0.05, 95% CI -0.16 to 0.05; very low-quality evidence). However, the risk of delayed systemic adverse effects with any of the three anti-VEGF drugs is not known.
Authors' conclusions: Implications for practice: Intravitreal bevacizumab/ranibizumab, when used as monotherapy, reduces the risk of refractive errors during childhood but does not reduce the risk of retinal detachment or recurrence of ROP in infants with type 1 ROP. While the intervention might reduce the risk of recurrence of ROP in infants with zone I ROP, it can potentially result in higher risk of recurrence requiring retreatment in those with zone II ROP. Intravitreal pegaptanib, when used in conjunction with laser therapy, reduces the risk of retinal detachment as well as the recurrence of ROP in infants with type 1 ROP. However, the quality of the evidence was very low to low for most outcomes due to risk of detection bias and other biases. The effects on other critical outcomes and, more importantly, the long-term systemic adverse effects of the drugs are not known. Insufficient data precludes strong conclusions favouring routine use of intravitreal anti-VEGF agents - either as monotherapy or in conjunction with laser therapy - in preterm infants with type 1 ROP.
Implications for research: Further studies are needed to evaluate the effect of anti-VEGF agents on structural and functional outcomes in childhood and delayed systemic effects including adverse neurodevelopmental outcomes.
Conflict of interest statement
Mari Jeeva Sankar: no conflict of interest
Jhuma Sankar: no conflict of interest
Parijat Chandra: no conflict of interest
Figures













Update of
-
Anti-vascular endothelial growth factor (VEGF) drugs for treatment of retinopathy of prematurity.Cochrane Database Syst Rev. 2016;2:CD009734. doi: 10.1002/14651858.CD009734.pub2. Epub 2016 Feb 27. Cochrane Database Syst Rev. 2016. Update in: Cochrane Database Syst Rev. 2018 Jan 08;1:CD009734. doi: 10.1002/14651858.CD009734.pub3. PMID: 26932750 Updated.
Similar articles
-
Anti-vascular endothelial growth factor (VEGF) drugs for treatment of retinopathy of prematurity.Cochrane Database Syst Rev. 2016;2:CD009734. doi: 10.1002/14651858.CD009734.pub2. Epub 2016 Feb 27. Cochrane Database Syst Rev. 2016. Update in: Cochrane Database Syst Rev. 2018 Jan 08;1:CD009734. doi: 10.1002/14651858.CD009734.pub3. PMID: 26932750 Updated.
-
Beta-blockers for prevention and treatment of retinopathy of prematurity in preterm infants.Cochrane Database Syst Rev. 2018 Mar 2;3(3):CD011893. doi: 10.1002/14651858.CD011893.pub2. Cochrane Database Syst Rev. 2018. PMID: 29499081 Free PMC article.
-
Anti-vascular endothelial growth factor for diabetic macular oedema: a network meta-analysis.Cochrane Database Syst Rev. 2017 Jun 22;6(6):CD007419. doi: 10.1002/14651858.CD007419.pub5. Cochrane Database Syst Rev. 2017. Update in: Cochrane Database Syst Rev. 2018 Oct 16;10:CD007419. doi: 10.1002/14651858.CD007419.pub6. PMID: 28639415 Free PMC article. Updated.
-
Anti-vascular endothelial growth factor combined with intravitreal steroids for diabetic macular oedema.Cochrane Database Syst Rev. 2018 Apr 18;4(4):CD011599. doi: 10.1002/14651858.CD011599.pub2. Cochrane Database Syst Rev. 2018. PMID: 29669176 Free PMC article.
-
Anti-vascular endothelial growth factor for choroidal neovascularisation in people with pathological myopia.Cochrane Database Syst Rev. 2016 Dec 15;12(12):CD011160. doi: 10.1002/14651858.CD011160.pub2. Cochrane Database Syst Rev. 2016. PMID: 27977064 Free PMC article.
Cited by
-
Preferred Treatment Patterns of Retinopathy of Prematurity: An International Survey.Pediatr Rep. 2024 Sep 13;16(3):816-822. doi: 10.3390/pediatric16030069. Pediatr Rep. 2024. PMID: 39311332 Free PMC article.
-
Predictors and ocular outcomes of rescue treatment in preterm infants with treated retinopathy of prematurity-a retrospective study.Eye (Lond). 2021 Jul;35(7):1937-1945. doi: 10.1038/s41433-020-01186-2. Epub 2020 Sep 21. Eye (Lond). 2021. PMID: 32958871 Free PMC article.
-
Irisin Attenuates Pathological Neovascularization in Oxygen-Induced Retinopathy Mice.Invest Ophthalmol Vis Sci. 2022 Jun 1;63(6):21. doi: 10.1167/iovs.63.6.21. Invest Ophthalmol Vis Sci. 2022. PMID: 35737379 Free PMC article.
-
Oral vitamin A supplementation for ROP prevention in VLBW preterm infants.Ital J Pediatr. 2020 Jun 3;46(1):77. doi: 10.1186/s13052-020-00837-0. Ital J Pediatr. 2020. PMID: 32493448 Free PMC article.
-
Efficacy comparison of anti-vascular endothelial growth factor drugs for the treatment of type 1 retinopathy of prematurity: A network meta-analysis of randomised controlled trials.Graefes Arch Clin Exp Ophthalmol. 2024 May;262(5):1409-1419. doi: 10.1007/s00417-023-06224-9. Epub 2023 Oct 10. Graefes Arch Clin Exp Ophthalmol. 2024. PMID: 37815595 Review.
References
References to studies included in this review
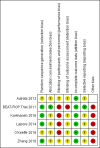
Autrata 2012 {published data only (unpublished sought but not used)}
-
- Autrata R, Krejčířová I, Šenková K, Holoušová M, Doležel Z, Borek I. Intravitreal pegaptanib combined with diode laser therapy for stage 3+ retinopathy of prematurity in zone I and posterior zone II. European Journal of Ophthalmology 2012;22(5):687‐94. [PUBMED: 22669848] - PubMed
BEAT‐ROP Trial 2011 {published data only (unpublished sought but not used)}
-
- Geloneck MM, Chuang AZ, Clark WL, Hunt MG, Norman AA, Packwood EA, et al. BEAT‐ROP Cooperative Group. Refractive outcomes following bevacizumab monotherapy compared with conventional laser treatment: a randomized clinical trial. JAMA Ophthalmology 2014;132(11):1327‐33. [PUBMED: 25103848] - PubMed
-
- NCT00622726. Bevacizumab Eliminates the Angiogenic Threat for Retinopathy of Prematurity (BEAT‐ROP). clinicaltrials.gov/ct2/show/NCT00622726 25 February 2008.
Karkhaneh 2016 {published data only}
Lepore 2014 {published data only}
-
- Lepore D, Quinn GE, Molle F, Baldascino A, Orazi L, Sammartino M, et al. Intravitreal bevacizumab versus laser treatment in type 1 retinopathy of prematurity: report on fluorescein angiographic findings. Ophthalmology 2014;121(11):2212‐9. [PUBMED: 25001158] - PubMed
O'Keeffe 2016 {published data only}
-
- O'Keeffe N, Murphy J, O'Keefe M, Lanigan B. Bevacizumab compared with diode laser in stage 3 posterior retinopathy of prematurity: a 5 year follow up. Irish Medical Journal 2016;109(2):355. [PUBMED: 27685689] - PubMed
Zhang 2016 {published data only}
-
- Zhang G, Yang M, Zeng J, Vakros G, Su K, Chen M, et al. Shenzhen Screening for Retinopathy of Prematurity Cooperative Group. Comparison of intravitreal injection of ranibuzumab versus laser therapy for zone II treatment‐requiring retinopathy of prematurity. Retina 2016 Aug 12 [Epub ahead of print]. [DOI: 10.1097/IAE.0000000000001241; PUBMED: 27529839] - DOI - PMC - PubMed
References to studies excluded from this review
Alyamac 2016 {published data only}
-
- Alyamac Sukgen E, Comez A, Kocluk Y, Cevher S. The process of retinal vascularization after anti‐VEGF treatment in retinopathy of prematurity: a comparison study between ranibizumab and bevacizumab. Ophthalmologica. Journal International d'Ophtalmologie [International Journal of Ophthalmology] 2016;236(3):139‐47. [DOI: 10.1159/000449530; PUBMED: 27682852] - DOI - PubMed
Araz‐Ersan 2015 {published data only}
-
- Araz‐Ersan B, Kir N, Tuncer S, Aydinoglu‐Candan O, Yildiz‐Inec D, Akdogan B, et al. Preliminary anatomical and neurodevelopmental outcomes of intravitreal bevacizumab as adjunctive treatment for retinopathy of prematurity. Current Eye Research 2015;40(6):585‐91. [DOI: 10.3109/02713683.2014.941070; PUBMED: 25025864] - DOI - PubMed
Chen 2015 {published data only}
-
- Chen SN, Lian I, Hwang YC, Chen YH, Chang YC, Lee KH, et al. Intravitreal anti‐vascular endothelial growth factor treatment for retinopathy of prematurity: comparison between ranibizumab and bevacizumab. Retina (Philadelphia, Pa.) 2015;35(4):667‐74. [PUBMED: 25462435] - PubMed
Erol 2015 {published data only}
Gunay 2016 {published data only}
Han 2016 {published data only}
Kabatas 2017 {published data only}
-
- Kabatas EU, Kurtul BE, Altiaylik Ozer P, Kabatas N. Comparison of intravitreal bevacizumab, intravitreal ranibizumab and laser photocoagulation for treatment of type 1 retinopathy of prematurity in Turkish preterm children. Current Eye Research 2017 Jan 27 [Epub ahead of print]. [DOI: 10.1080/02713683.2016.1264607; PUBMED: 28128986] - DOI - PubMed
Lien 2016 {published data only}
Lin 2016 {published data only}
Morin 2016 {published data only}
-
- Morin J, Luu TM, Superstein R, Ospina LH, Lefebvre F, Simard MN, et al. Canadian Neonatal Network and the Canadian Neonatal Follow‐Up Network Investigators. Neurodevelopmental outcomes following bevacizumab injections for retinopathy of prematurity. Pediatrics 2016;137(4):e20153218. [DOI: 10.1542/peds.2015-3218; PUBMED: 27244705] - DOI - PubMed
References to studies awaiting assessment
Autrata 2012a {published data only}
-
- Autrata R, Senkova K, Holousova M, Krejcirova I, Dolezel Z, Borek I. Effects of intravitreal pegaptanib or bevacizumab and laser in treatment of threshold retinopathy of prematurity in zone I and posterior zone II ‐ four years results [Prinos intravitrealni aplikace anti‐VEGF preparatu v lecbe prahoveho stadia ROP 3+ v zone I‐II: vysledky ctyrlete studie]. Ceska a Slovenska Oftalmologie 2012;68(1):29‐36. - PubMed
Kong 2015 {published data only}
-
- Kong L, Bhatt AR, Demny AB, Coats DK, Li A, Rahman EZ, et al. Pharmacokinetics of bevacizumab and its effects on serum VEGF and IGF‐1 in infants with retinopathy of prematurity. Investigative Ophthalmology & Visual Science 2015;56(2):956‐61. [DOI: 10.1167/iovs.14-15842; PUBMED: 25613938] - DOI - PubMed
Additional references
Aiello 1995
-
- Aiello LP, Pierce EA, Foley ED, Takagi H, Chen H, Riddle L, et al. Suppression of retinal neovascularization in vivo by inhibition of vascular endothelial growth factor (VEGF) using soluble VEGF‐receptor chimeric proteins. Proceedings of the National Academy of Sciences of the United States of America 1995;92(23):10457‐61. [PUBMED: 7479819] - PMC - PubMed
American Academy of Pediatrics 2006
-
- Section on Ophthalmology American Academy of Pediatrics, American Academy of Ophthalmology, American Association for Pediatric Ophthalmology and Strabismus. Screening examination of premature infants for retinopathy of prematurity. Pediatrics 2006;117(2):572‐6. [DOI: 10.1542/peds.2005-2749; PUBMED: 16452383] - DOI - PubMed
Andersen 1999
Ashton 1953
Ashton 1954
Committee for Classification of ROP 1984
-
- The Committee for the Classification of Retinopathy of Prematurity. An international classification of retinopathy of prematurity. Archives of Ophthalmology 1984;102(8):1130‐4. [PUBMED: 6547831] - PubMed
Deeks 2011
-
- Deeks JJ, Higgins JPT, Altman DG (editors). Chapter 9: Analysing data and undertaking meta‐analyses. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
ETROP Group 2003
-
- Early Treatment for Retinopathy Of Prematurity Cooperative Group. Revised indications for the treatment of retinopathy of prematurity: results of the Early Treatment for Retinopathy Of Prematurity randomized trial. Archives of Ophthalmology 2003;121(12):1684‐94. [PUBMED: 14662586] - PubMed
Gilbert 2005
-
- Gilbert C, Fielder A, Gordillo L, Quinn G, Semiglia R, Visintin P, et al. Characteristics of infants with severe retinopathy of prematurity in countries with low, moderate, and high levels of development: implications for screening programs. Pediatrics 2005;115(5):e518‐25. [PUBMED: 15805336] - PubMed
Gilbert 2008
GRADEpro GDT [Computer program]
-
- McMaster University (developed by Evidence Prime). Available at gradepro.org. GRADEpro GDT. Version (accessed 5 December 2017). Hamilton (ON): McMaster University (developed by Evidence Prime). Available at gradepro.org, 2015.
Higgins 2003
Higgins 2011
-
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Higgins 2011a
-
- Higgins JPT, Deeks JJ, Altman DG (editors). Chapter 16: Special topics in statistics. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Kong 2008
-
- Kong L, Mintz‐Hittner HA, Penland RL, Kretzer FL, Chevez‐Barrios P. Intravitreous bevacizumab as anti‐vascular endothelial growth factor therapy for retinopathy of prematurity: a morphologic study. Archives of Ophthalmology 2008;126(8):1161‐3. [PUBMED: 18695118] - PubMed
Lashkari 2000
-
- Lashkari K, Hirose T, Yazdany J, McMeel JW, Kazlauskas A, Rahimi N. Vascular endothelial growth factor and hepatocyte growth factor levels are differentially elevated in patients with advanced retinopathy of prematurity. American Journal of Pathology 2000;156(4):1337‐44. [PUBMED: 10751359] - PMC - PubMed
Law 2010
-
- Law JC, Recchia FM, Morrison DG, Donahue SP, Estes RL. Intravitreal bevacizumab as adjunctive treatment for retinopathy of prematurity. Journal of AAPOS 2010;14(1):6‐10. [PUBMED: 20227614] - PubMed
Mantagos 2009
Micieli 2009
-
- Micieli JA, Surkont M, Smith AF. A systematic analysis of the off‐label use of bevacizumab for severe retinopathy of prematurity. American Journal of Ophthalmology 2009;148(4):536‐43.e2. [PUBMED: 19660736] - PubMed
Mintz‐Hittner 2008
-
- Mintz‐Hittner HA, Kuffel RR Jr. Intravitreal injection of bevacizumab (Avastin) for treatment of stage 3 retinopathy of prematurity in zone I or posterior zone II. Retina 2008;28(6):831‐8. [PUBMED: 18536599] - PubMed
Nicoara 2016
-
- Nicoară SD, Ștefănuţ AC, Nascutzy C, Zaharie GC, Toader LE, Drugan TC. Regression rates following the treatment of aggressive posterior retinopathy of prematurity with bevacizumab versus laser: 8‐year retrospective analysis. Medical Science Monitor 2016;22:1192‐209. [PUBMED: 27062023] - PMC - PubMed
Palmer 1997
-
- Palmer EA. What have we learned about retinopathy of prematurity during the past ten years? Progress in retinopathy of prematurity. The International Symposium on Retinopathy of Prematurity; 1997; Taormina, Italy. Amsterdam/New York: Kugler Publications, 1997.
Pertl 2015
Review Manager 2014 [Computer program]
-
- Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager 5 (RevMan 5). Version 5.3. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Schünemann 2013
-
- Schünemann H, Brożek J, Guyatt G, Oxman A (editors). Handbook for grading the quality of evidence and the strength of recommendations using the GRADE approach (updated October 2013). GRADE Working Group, 2013. gdt.guidelinedevelopment.org/app/handbook/handbook.html (accessed prior to 12 December 2017).
Shah 2007
-
- Shah PK, Narendran V, Tawansy KA, Raghuram A, Narendran K. Intravitreal bevacizumab (Avastin) for post laser anterior segment ischemia in aggressive posterior retinopathy of prematurity. Indian Journal of Ophthalmology 2007;55(1):75‐6. [PUBMED: 17189897] - PubMed
Smith 2003
-
- Smith LE. Pathogenesis of retinopathy of prematurity. Seminars in Neonatology 2003;8(6):469‐73. [PUBMED: 15001119] - PubMed
Sterne 2011
-
- Sterne JAC, Egger M, Moher D (editors). Chapter 10: Addressing reporting biases. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Tasman 2006
-
- Tasman W, Patz A, McNamara JA, Kaiser RS, Trese MT, Smith BT. Retinopathy of prematurity: the life of a lifetime disease. American Journal of Ophthalmology 2006;141(1):167‐74. [PUBMED: 16386993] - PubMed
Ueta 2009
-
- Ueta T, Yanagi Y, Tamaki Y, Yamaguchi T. Cerebrovascular accidents in ranibizumab. Ophthalmology 2009; Vol. 116, issue 2:362. [PUBMED: 19187826] - PubMed
Wu 2013
-
- Wu WC, Kuo HK, Yeh PT, Yang CM, Lai CC, Chen SN. An updated study of the use of bevacizumab in the treatment of patients with prethreshold retinopathy of prematurity in Taiwan. American Journal of Ophthalmology 2013;155(1):150‐8. [PUBMED: 22967867] - PubMed
References to other published versions of this review
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

