Dissection of affinity captured LINE-1 macromolecular complexes
- PMID: 29309035
- PMCID: PMC5821459
- DOI: 10.7554/eLife.30094
Dissection of affinity captured LINE-1 macromolecular complexes
Abstract
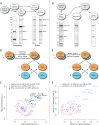
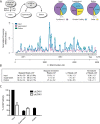
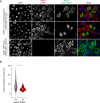
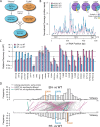
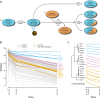
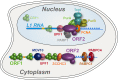
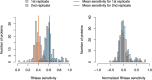
Long Interspersed Nuclear Element-1 (LINE-1, L1) is a mobile genetic element active in human genomes. L1-encoded ORF1 and ORF2 proteins bind L1 RNAs, forming ribonucleoproteins (RNPs). These RNPs interact with diverse host proteins, some repressive and others required for the L1 lifecycle. Using differential affinity purifications, quantitative mass spectrometry, and next generation RNA sequencing, we have characterized the proteins and nucleic acids associated with distinctive, enzymatically active L1 macromolecular complexes. Among them, we describe a cytoplasmic intermediate that we hypothesize to be the canonical ORF1p/ORF2p/L1-RNA-containing RNP, and we describe a nuclear population containing ORF2p, but lacking ORF1p, which likely contains host factors participating in target-primed reverse transcription.
Keywords: LINE-1; affinity proteomics; biochemistry; chemical biology; computational biology; human; interactome; retrotransposon; ribonucleoprotein; systems biology.
© 2018, Taylor et al.
Conflict of interest statement
MT, IA, KM, PM, HJ, EA, AW, SB, DI, GE, KB, DF, BC, DA, MR, JB, JL No competing interests declared
Figures



Comment in
-
On the move.Elife. 2018 Feb 21;7:e34901. doi: 10.7554/eLife.34901. Elife. 2018. PMID: 29465398 Free PMC article.
Similar articles
-
Characterization of LINE-1 ribonucleoprotein particles.PLoS Genet. 2010 Oct 7;6(10):e1001150. doi: 10.1371/journal.pgen.1001150. PLoS Genet. 2010. PMID: 20949108 Free PMC article.
-
Characterization of L1-Ribonucleoprotein Particles.Methods Mol Biol. 2016;1400:311-38. doi: 10.1007/978-1-4939-3372-3_20. Methods Mol Biol. 2016. PMID: 26895062 Free PMC article.
-
A potential role for the nucleolus in L1 retrotransposition.Hum Mol Genet. 2004 May 15;13(10):1041-8. doi: 10.1093/hmg/ddh118. Epub 2004 Mar 17. Hum Mol Genet. 2004. PMID: 15028673
-
Protein-nucleic acid interactions of LINE-1 ORF1p.Semin Cell Dev Biol. 2019 Feb;86:140-149. doi: 10.1016/j.semcdb.2018.03.019. Epub 2018 Mar 31. Semin Cell Dev Biol. 2019. PMID: 29596909 Free PMC article. Review.
-
Nucleic acid chaperone properties of ORF1p from the non-LTR retrotransposon, LINE-1.RNA Biol. 2010 Nov-Dec;7(6):706-11. doi: 10.4161/rna.7.6.13766. Epub 2010 Nov 1. RNA Biol. 2010. PMID: 21045547 Free PMC article. Review.
Cited by
-
ZCCHC3 is a stress granule zinc knuckle protein that strongly suppresses LINE-1 retrotransposition.PLoS Genet. 2023 Jul 5;19(7):e1010795. doi: 10.1371/journal.pgen.1010795. eCollection 2023 Jul. PLoS Genet. 2023. PMID: 37405998 Free PMC article.
-
Condensation of LINE-1 is critical for retrotransposition.Elife. 2023 Apr 28;12:e82991. doi: 10.7554/eLife.82991. Elife. 2023. PMID: 37114770 Free PMC article.
-
Opposing roles of pseudokinases NRBP1 and NRBP2 in regulating L1 retrotransposition.Nat Commun. 2025 Jul 11;16(1):6327. doi: 10.1038/s41467-025-61626-z. Nat Commun. 2025. PMID: 40645931 Free PMC article.
-
Hepatitis C virus infection restricts human LINE-1 retrotransposition in hepatoma cells.PLoS Pathog. 2021 Apr 19;17(4):e1009496. doi: 10.1371/journal.ppat.1009496. eCollection 2021 Apr. PLoS Pathog. 2021. PMID: 33872335 Free PMC article.
-
Cell fitness screens reveal a conflict between LINE-1 retrotransposition and DNA replication.Nat Struct Mol Biol. 2020 Feb;27(2):168-178. doi: 10.1038/s41594-020-0372-1. Epub 2020 Feb 10. Nat Struct Mol Biol. 2020. PMID: 32042151 Free PMC article.
References
-
- Altukhov I. LINE-1. 1e90c62Bitbucket. 2017 https://bitbucket.org/altukhov/line-1/
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

