Influenza-specific lung-resident memory T cells are proliferative and polyfunctional and maintain diverse TCR profiles
- PMID: 29309047
- PMCID: PMC5785253
- DOI: 10.1172/JCI96957
Influenza-specific lung-resident memory T cells are proliferative and polyfunctional and maintain diverse TCR profiles
Abstract
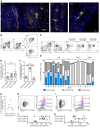
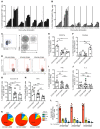
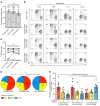
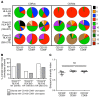
The human lung harbors a large population of resident memory T cells (Trm cells). These cells are perfectly positioned to mediate rapid protection against respiratory pathogens such as influenza virus, a highly contagious respiratory pathogen that continues to be a major public health burden. Animal models show that influenza-specific lung CD8+ Trm cells are indispensable for crossprotection against pulmonary infection with different influenza virus strains. However, it is not known whether influenza-specific CD8+ Trm cells present within the human lung have the same critical role in modulating the course of the disease. Here, we showed that human lung contains a population of CD8+ Trm cells that are highly proliferative and have polyfunctional progeny. We observed that different influenza virus-specific CD8+ T cell specificities differentiated into Trm cells with varying efficiencies and that the size of the influenza-specific CD8+ T cell population persisting in the lung directly correlated with the efficiency of differentiation into Trm cells. To our knowledge, we provide the first ex vivo dissection of paired T cell receptor (TCR) repertoires of human influenza-specific CD8+ Trm cells. Our data reveal diverse TCR profiles within the human lung Trm cells and a high degree of clonal sharing with other CD8+ T cell populations, a feature important for effective T cell function and protection against the generation of viral-escape mutants.
Keywords: Adaptive immunity; Immunology; Infectious disease; Influenza; T cells.
Conflict of interest statement
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

