Hepatitis B virus-specific T cells associate with viral control upon nucleos(t)ide-analogue therapy discontinuation
- PMID: 29309050
- PMCID: PMC5785266
- DOI: 10.1172/JCI92812
Hepatitis B virus-specific T cells associate with viral control upon nucleos(t)ide-analogue therapy discontinuation
Abstract
Background: The clinical management of chronic hepatitis B virus (HBV) patients is based exclusively on virological parameters that cannot independently determine in which patients nucleos(t)ide-analogue (NUC) therapy can be safely discontinued. NUCs efficiently suppress viral replication, but do not eliminate HBV. Thus, therapy discontinuation can be associated with virological and biochemical relapse and, consequently, therapy in the majority is life-long.
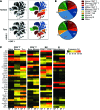
Methods: Since antiviral immunity is pivotal for HBV control, we investigated potential biomarkers for the safe discontinuation of NUCs within immune profiles of chronic HBV patients by utilizing traditional immunological assays (ELISPOT, flow cytometry) in conjunction with analyses of global non-antigen-specific immune populations (NanoString and CyTOF). Two distinct cohorts of 19 and 27 chronic HBV patients, respectively, were analyzed longitudinally prior to and after discontinuation of 2 different NUC therapy strategies.
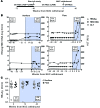
Results: Absence of hepatic flares following discontinuation of NUC treatment correlated with the presence, during NUC viral suppression, of HBV core and polymerase-specific T cells that were contained within the ex vivo PD-1+ population.
Conclusions: This study identifies the presence of functional HBV-specific T cells as a candidate immunological biomarker for safe therapy discontinuation in chronic HBV patients. Furthermore, the persistent and functional antiviral activity of PD-1+ HBV-specific T cells highlights the potential beneficial role of the expression of T cell exhaustion markers during human chronic viral infection.
Funding: This work was funded by a Singapore Translational Research Investigator Award (NMRC/STaR/013/2012), the Eradication of HBV TCR Program (NMRC/TCR/014-NUHS/2015), the Singapore Immunology Network, the Wellcome Trust (107389/Z/15/Z), and a Barts and The London Charity (723/1795) grant.
Keywords: Drug therapy; Hepatitis; Hepatology; Immunology; T cells.
Conflict of interest statement
Figures



Comment in
-
Unravelling the fate of functional PD1+ T cells in chronic viral hepatitis.J Clin Invest. 2018 Feb 1;128(2):573-576. doi: 10.1172/JCI99035. Epub 2018 Jan 8. J Clin Invest. 2018. PMID: 29309052 Free PMC article.
-
Viral hepatitis: Biomarker for HBV therapy discontinuation.Nat Rev Gastroenterol Hepatol. 2018 Mar;15(3):132. doi: 10.1038/nrgastro.2018.6. Epub 2018 Jan 24. Nat Rev Gastroenterol Hepatol. 2018. PMID: 29362470 No abstract available.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

