Unravelling the fate of functional PD1+ T cells in chronic viral hepatitis
- PMID: 29309052
- PMCID: PMC5785249
- DOI: 10.1172/JCI99035
Unravelling the fate of functional PD1+ T cells in chronic viral hepatitis
Abstract
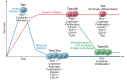
Hepatitis B virus (HBV) infection can be managed clinically with nucleos(t)ide therapy, which suppresses viral replication; however, these drugs must often be used long term, as they are unable to fully eliminate the virus. For many patients, discontinuation of treatment results in viral resurgence and hepatic flare, and there is not a reliable way to identify those individuals that can be successfully taken off nucleos(t)ide therapy. In this issue of the JCI, Rivino and colleagues report on their use of a multipronged approach to investigate potential biomarkers indicative of HBV-infected patients who can safely stop nucleos(t)ide therapy. The authors identified a population of HBV-specific, PD1-positive T cells that was present in HBV-infected patients who successfully discontinued treatment without hepatic flare, but not in those that developed flare upon treatment cessation. Together, these results support the concept that PD1+ cells may play an important role in viral control, the further evaluation of this T cell subset in preventing hepatic flare, and the development of assays to better detect this PD1+ T cell population in HBV-infected patients on nucleos(t)ide therapy.
Conflict of interest statement
Figures
Comment on
-
Hepatitis B virus-specific T cells associate with viral control upon nucleos(t)ide-analogue therapy discontinuation.J Clin Invest. 2018 Feb 1;128(2):668-681. doi: 10.1172/JCI92812. Epub 2018 Jan 8. J Clin Invest. 2018. PMID: 29309050 Free PMC article.
References
-
- World Health Organization. Global Hepatitis Report 2017. WHO Website. http://www.who.int/hepatitis/publications/global-hepatitis-report2017/en/ Accessed December 12, 2017.
-
- Liu Z, Liu F, Wang L, Liu Y, Zhang M, Li T. Clinical characteristics and outcomes of patients with recurrent chronic hepatitis B after nucleos(t)ide analog withdrawal with stringent cessation criteria: A prospective cohort study. Hepatol Res. 2017;47(10):1000–1007. doi: 10.1111/hepr.12836. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

