Mechanism of intersubunit ketosynthase-dehydratase interaction in polyketide synthases
- PMID: 29309054
- PMCID: PMC5846730
- DOI: 10.1038/nchembio.2549
Mechanism of intersubunit ketosynthase-dehydratase interaction in polyketide synthases
Abstract
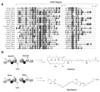
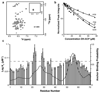
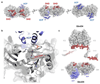
Modular polyketide synthases (PKSs) produce numerous structurally complex natural products that have diverse applications in medicine and agriculture. PKSs typically consist of several multienzyme subunits that utilize structurally defined docking domains (DDs) at their N and C termini to ensure correct assembly into functional multiprotein complexes. Here we report a fundamentally different mechanism for subunit assembly in trans-acyltransferase (trans-AT) modular PKSs at the junction between ketosynthase (KS) and dehydratase (DH) domains. This mechanism involves direct interaction of a largely unstructured docking domain (DD) at the C terminus of the KS with the surface of the downstream DH. Acyl transfer assays and mechanism-based crosslinking established that the DD is required for the KS to communicate with the acyl carrier protein appended to the DH. Two distinct regions for binding of the DD to the DH were identified using NMR spectroscopy, carbene footprinting, and mutagenesis, providing a foundation for future elucidation of the molecular basis for interaction specificity.
Conflict of interest statement
The authors declare no competing financial interests.
Figures


References
-
- Hertweck C. The biosynthetic logic of polyketide diversity. Angew Chem Int Ed Engl. 2009;48:4688–716. - PubMed
-
- Keatinge-Clay AT. The structures of type I polyketide synthases. Nat Prod Rep. 2012;29:1050–1073. - PubMed
-
- Richter CD, Nietlispach D, Broadhurst RW, Weissman KJ. Multienzyme docking in hybrid megasynthetases. Nat Chem Biol. 2008;4:75–81. - PubMed
-
- Weissman KJ, Muller R. Protein-protein interactions in multienzyme megasynthetases. Chembiochem. 2008;9:826–848. - PubMed
-
- Fischbach MA, Walsh CT. Assembly-line enzymology for polyketide and nonribosomal peptide antibiotics: Logic, machinery, and mechanisms. Chem Rev. 2006;106:3468–3496. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- 639907/ERC_/European Research Council/International
- BB/L021692/1/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
- BB/L022761/1/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
- BB/M017982/1/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
LinkOut - more resources
Full Text Sources
Other Literature Sources

