Oxidative stress damages rRNA inside the ribosome and differentially affects the catalytic center
- PMID: 29309687
- PMCID: PMC5829716
- DOI: 10.1093/nar/gkx1308
Oxidative stress damages rRNA inside the ribosome and differentially affects the catalytic center
Abstract
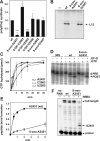
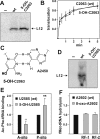
Intracellular levels of reactive oxygen species (ROS) increase as a consequence of oxidative stress and represent a major source of damage to biomolecules. Due to its high cellular abundance RNA is more frequently the target for oxidative damage than DNA. Nevertheless the functional consequences of damage on stable RNA are poorly understood. Using a genome-wide approach, based on 8-oxo-guanosine immunoprecipitation, we present evidence that the most abundant non-coding RNA in a cell, the ribosomal RNA (rRNA), is target for oxidative nucleobase damage by ROS. Subjecting ribosomes to oxidative stress, we demonstrate that oxidized 23S rRNA inhibits the ribosome during protein biosynthesis. Placing single oxidized nucleobases at specific position within the ribosome's catalytic center by atomic mutagenesis resulted in markedly different functional outcomes. While some active site nucleobases tolerated oxidative damage well, oxidation at others had detrimental effects on protein synthesis by inhibiting different sub-steps of the ribosomal elongation cycle. Our data provide molecular insight into the biological consequences of RNA oxidation in one of the most central cellular enzymes and reveal mechanistic insight on the role of individual active site nucleobases during translation.
Figures

References
-
- Hofer T., Seo A.Y., Prudencio M., Leeuwenburgh C.. A method to determine RNA and DNA oxidation simultaneously by HPLC-ECD: greater RNA than DNA oxidation in rat liver after doxorubicin administration. Biol. Chem. 2006; 387:103–111. - PubMed
-
- Küpfer P., Leumann C.. Erdmann VA. Chemical Biology of Nucleic Acids. 2014; 5:Berlin, Heidelberg: Springer-Verlag; 75–94.
-
- Hofer T., Badouard C., Bajak E., Ravanat J.L., Mattsson A., Cotgreave I.A.. Hydrogen peroxide causes greater oxidation in cellular RNA than in DNA. Biol. Chem. 2005; 386:333–337. - PubMed
-
- Poulsen H.E., Specht E., Broedbaek K., Henriksen T., Ellervik C., Mandrup-Poulsen T., Tonnesen M., Nielsen P.E., Andersen H.U., Weimann A.. RNA modifications by oxidation: a novel disease mechanism?. Free Radic. Biol. Med. 2012; 52:1353–1361. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

