The ubiquitin ligase ITCH coordinates small intestinal epithelial homeostasis by modulating cell proliferation, differentiation, and migration
- PMID: 29309986
- PMCID: PMC5826883
- DOI: 10.1016/j.diff.2017.12.003
The ubiquitin ligase ITCH coordinates small intestinal epithelial homeostasis by modulating cell proliferation, differentiation, and migration
Abstract
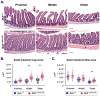
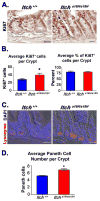
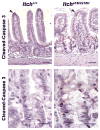
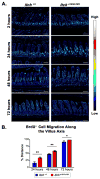
Maintenance of the intestinal mucosa is driven by local signals that coordinate epithelial proliferation, differentiation, and turnover in order to separate antigenic luminal contents from the host's immune system. Breaches in this barrier promote gastrointestinal pathologies ranging from inflammatory bowel disease to cancer. The ubiquitin ligase ITCH is known to regulate immune responses, and loss of function of ITCH has been associated with gastrointestinal inflammatory disorders, particularly in the colon. However, the small intestine appears to be spared from this pathology. Here we explored the physiological mechanism that underlies the preservation of mucosal homeostasis in the small intestine in mice lacking ITCH (Itcha18H/a18H). Histological analysis of the small intestines from young adult mice revealed architectural changes in animals deficient for ITCH, including villus blunting with cell crowding, crypt expansion, and thickening of the muscularis propria relative to age-matched mice sufficient for ITCH. These differences were more prominent in the distal part of the small intestine and were not dependent upon lymphoid cells. Underlying the observed changes in the epithelium were expansion of the Ki67+ proliferating transit amplifying progenitor population and increased numbers of terminally differentiated mucus-secreting goblet and anti-microbial producing Paneth cells, which are both important in controlling local inflammation in the small intestine and are known to be dysregulated in inflammatory bowel disease. Homeostasis in the small intestine of Itcha18H/a18H animals was maintained by increased cell turnover, including accelerated migration of epithelial cells along the crypt-villus axis and increased apoptosis of epithelial cells at the crypt-villus junction. Consistent with this enhanced turnover, Itcha18H/a18H mice carrying the Min mutation (Itcha18H/a18H; ApcMin/+) displayed a 76% reduction in tumor burden as compared to ApcMin/+ littermates with normal levels of ITCH. These findings highlight the role of ITCH as an important modulator of intestinal epithelial homeostasis.
Keywords: Cell migration; Colorectal cancer; E3 ubiquitin ligase; Epithelial differentiation; ITCH; Intestinal homeostasis.
Copyright © 2018 International Society of Differentiation. Published by Elsevier B.V. All rights reserved.
Figures



References
-
- Agace WW, McCoy KD. Regionalized development and maintenance of the intestinal adaptive immune landscape. Immunity. 2017;46:532–548. - PubMed
-
- Barker N. Adult intestinal stem cells: critical drivers of epithelial homeostasis and regeneration. Nat Rev Mol Cell Biol. 2014;15:19–33. - PubMed
-
- Barker N, van Es JH, Kuipers J, Kujala P, van den Born M, Cozijnsen M, Haegebarth A, Korving J, Begthel H, Peters PJ, Clevers H. Identification of stem cells in small intestine and colon by marker gene Lgr5. Nature. 2007;449:1003–1007. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

