Trastuzumab without chemotherapy in the adjuvant treatment of breast cancer: subgroup results from a large observational study
- PMID: 29310623
- PMCID: PMC5759796
- DOI: 10.1186/s12885-017-3857-5
Trastuzumab without chemotherapy in the adjuvant treatment of breast cancer: subgroup results from a large observational study
Abstract
Background: The topic of trastuzumab therapy without chemotherapy in early breast cancer (EBC) has been repeatedly discussed at international consensus meetings, but is compromised by the lack of solid evidence from clinical studies.
Methods: An observational study database of patients with EBC receiving trastuzumab-containing (neo)adjuvant therapy was screened to identify those patients who did not receive cytostatic agents.
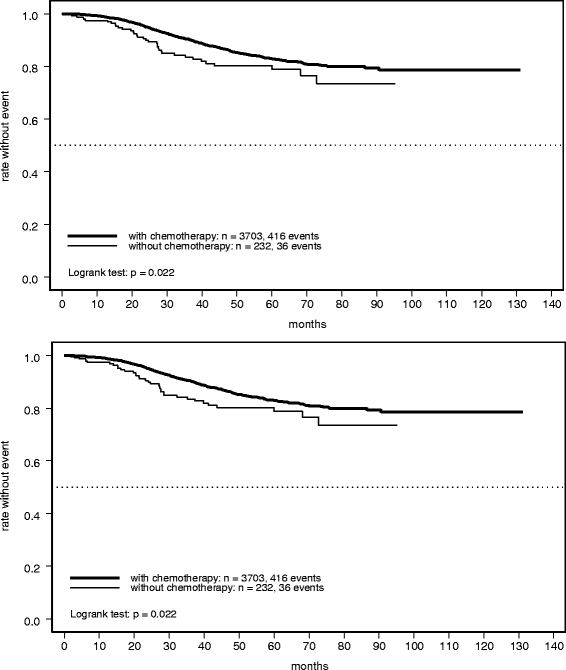
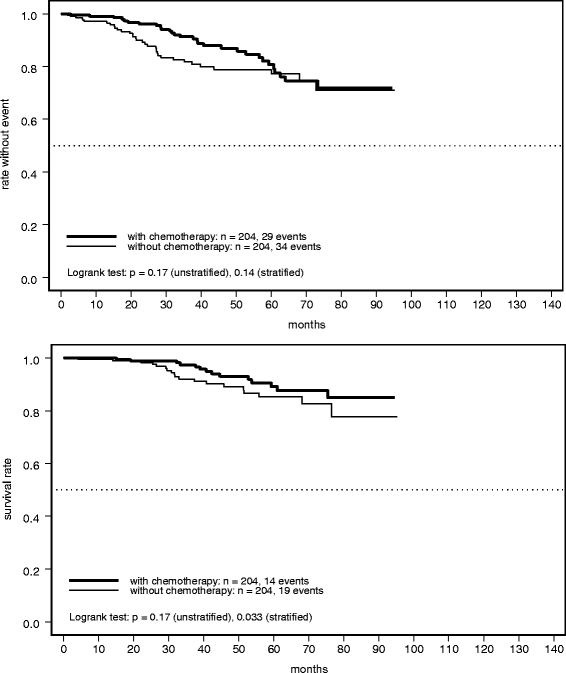
Results: Of 3935 patients, 232 (6%) were identified who received no chemotherapy, being characterized by older age, worse performance status, and/or less aggressive histology. Relapse-free survival in this cohort was 84% (95% confidence interval [CI] 78-89%) at 3 years and 80% (95% CI 74-87%) at 5 years. However, these rates were significantly worse than those in the group of patients who received chemotherapy (hazard ratio 1.49; 95% CI 1.06-2.09; P = 0.022). A similar pattern was observed for overall survival, with marginally non-significant inferiority in the group receiving no chemotherapy (hazard ratio 1.56; 95% CI 1.00-2.44; P = 0.052). Survival rates in patients receiving no chemotherapy were 93% (95% CI 88-97%) and 87% (95% CI 81-93%) at 3 and 5 years, respectively. These findings were confirmed by a propensity score analysis accounting for selection bias.
Conclusions: Trastuzumab plus chemotherapy should remain the preferred option in all patients with HER2-positive EBC with an indication for adjuvant treatment. However, a limited proportion of patients will need an alternative treatment approach, either because of contraindications or the patient's preference. In these selected patients, trastuzumab monotherapy, eventually combined with endocrine agents, might be a reasonable option offering favorable long-term outcomes by addressing the high-risk profile associated with HER2-positive disease.
Keywords: HER2-positive; Monotherapy; Overall survival; Propensity score analysis; Relapse-free survival.
Conflict of interest statement
Ethics approval and consent to participate
This was an observational study in which physicians’ choices were guided by drug registration status and treatment guidelines (rather than the observation protocol). As the study was started prior to 2007, it was in agreement with the German FSA Codex and the German Arzneimittelgesetz Amendment 12, that there was no need/requirement for ethics committee approval or written informed consent. For non-interventional studies started in 2007 or later, the FSA Codex asks for submission to the ethics committee (recommended) and to the regulators [
Consent for publication
Not applicable.
Competing interests
Peter Dall: Roche Pharma AG, Novartis, Astra Zeneca (Honoraria received), Roche Pharma AG (Travel, accommodations or expenses); Carsten Hielscher: Roche Pharma AG, Celgene, Oncovis (Honoraria received); Pfizer, Oncovis (Travel, accommodations or expenses); Nicolas Schleif: Roche Pharma AG (Employment); Axel Hinke: Roche Pharma AG (Honoraria received). The other authors indicated no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures
References
-
- Perez EA, Romond EH, Suman VJ, et al. Four-year follow-up of trastuzumab plus adjuvant chemotherapy for operable human epidermal growth factor receptor 2-positive breast cancer: joint analysis of data from NCCTG N9831 and NSABP B-31. J Clin Oncol. 2011;29:3366–3373. doi: 10.1200/JCO.2011.35.0868. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

