Impact of collaborative pharmaceutical care on in-patients' medication safety: study protocol for a stepped wedge cluster randomized trial (MEDREV study)
- PMID: 29310711
- PMCID: PMC5759250
- DOI: 10.1186/s13063-017-2412-7
Impact of collaborative pharmaceutical care on in-patients' medication safety: study protocol for a stepped wedge cluster randomized trial (MEDREV study)
Abstract
Background: Clinical pharmaceutical care has long played an important role in the improvement of healthcare safety. Pharmaceutical care is a collaborative care approach, implicating all the actors of the medication circuit in order to prevent and correct drug-related problems that can lead to adverse drug events. The collaborative pharmaceutical care performed during patients' hospitalization requires two mutually reinforcing activities: medication reconciliation and medication review. Until now, the impact of the association of these two activities has not been clearly studied.
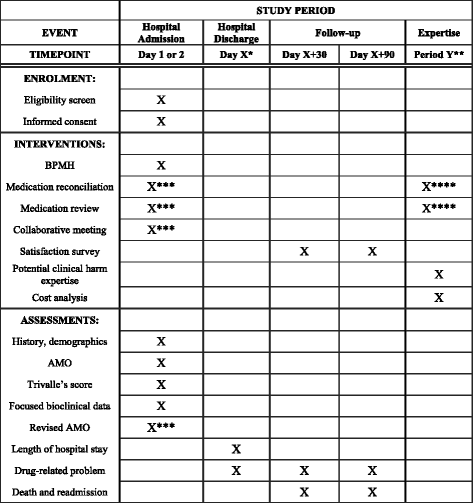
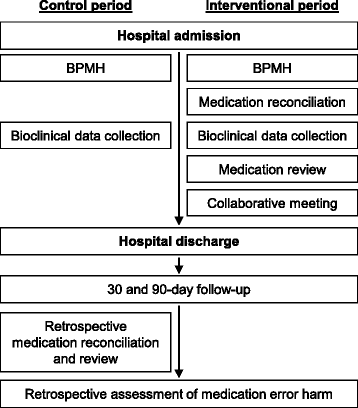
Methods: This is a multicentric stepped wedge randomized study involving six care units from six French University Hospitals (each unit corresponding to a cluster) over seven consecutive 14-day periods. Each hospital unit will start with a control period and switch to an experimental period after a randomized number of 14-day periods. Patients aged at least 65 years hospitalized in one of the participating care units and having given their consent to be called for a 30-day and 90-day follow-up can be enrolled. For each 14-day period, 15 patients will be recruited in each care unit to obtain a total of 630 patients enrolled in all centers. Patients with a hospital stay of more than 21 days will be excluded. During the control period, there will be no clinical pharmacist in the care unit, whereas during the experimental period a clinical pharmacist will perform medication reconciliation and review with the healthcare team. The primary outcome will assess the impact of collaborative pharmaceutical care on preventable medication error rate. The secondary outcomes will evaluate the clinical impact of the strategy, the acceptance rate of pharmaceutical interventions, the induced and avoided costs of the strategy (cost-consequence analysis), and the healthcare team's satisfaction.
Discussion: This study will assess the impact of collaborative pharmaceutical care associating medication reconciliation and review at patient admission to hospital in terms of preventable medication error rate and costs. This activity will prevent and correct medication errors arising earlier in the hospitalization.
Trial registration: ClinicalTrials.gov, NCT02598115 . Registered on 4 November 2015.
Keywords: Drug safety; Drug-related problem; Hospital pharmacist; Medication reconciliation; Medication review; Pharmaceutical care; Stepped wedge study.
Conflict of interest statement
Authors’ information
GLB, CC, CRM, PC: co-investigator, member of the monitoring committee. SB: biostatistician. SA: member of the scientific committee, vice-president of the French Society of Geriatrics and Gerontology. RC: co-investigator, member of the monitoring committee, vice-president of SFPC. PL: member of the scientific committee, head of the clinical research department. BL: member of the scientific committee, MED’REC French coordinator. RV: member of the scientific committee, president of SFPC. BA: responsible for the scientific committee, president of the scientific committee of SFPC. MEDREV Working Group: participating health professionals. PB: member of the monitoring committee. JMK: member of the scientific committee, principal investigator.
Ethics approval and consent to participate
Nîmes University Hospital Center is the promoter and is responsible for all the administrative measures. The local ethics committee (the Third Southern Mediterranean Protection to Persons Committee) approved the study for all centers. According to French law, all the patients or their legal representative will be verbally informed during the inclusion visit and will receive an information form. The patient can refuse to participate at any time. The French committees for data handling (Advisory Committee on Information Processing in Material Research in the Field of Health and National Commission on Computer Technology and Freedom) approved the study. This trial was registered at ClinicalTrials.gov (number NCT02598115 on 4 November 2015: Impact of collaborative pharmaceutical care on hospital admission drug prescriptions for patients 65 years of age and older (MEDREV)).
Any substantial change is subject to a written amendment submitted to the promoter, who must obtain a favorable opinion from the PPC and an authorization from the competent authority before implementation. All amendments to the protocol must be made known to all investigators involved in the research.
Consent for publication
All authors read and approved the final manuscript.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures

References
-
- Michel P, Lathelize M, Quenon JL, Bru-Sonnet R, Domecq S, Kret M. [Comparison of two National Studies on Severe Adverse Events induced by healthcare conducted in 2004 and 2009. Final Report by DREES (Health Ministry). 2011. http://drees.solidarites-sante.gouv.fr/IMG/pdf/serieetud109.pdf. Accessed Oct 2012.
-
- Apretna E, Haramburu F, Taboulet F, Bégaud B. [Medical and socio-economical impact of drug-induced adverse reactions] Presse Medicale Paris Fr 1983. 2005;34(4):271–6. - PubMed
Publication types
MeSH terms
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

