Characterization of glycosylphosphatidylinositol biosynthesis defects by clinical features, flow cytometry, and automated image analysis
- PMID: 29310717
- PMCID: PMC5759841
- DOI: 10.1186/s13073-017-0510-5
Characterization of glycosylphosphatidylinositol biosynthesis defects by clinical features, flow cytometry, and automated image analysis
Abstract
Background: Glycosylphosphatidylinositol biosynthesis defects (GPIBDs) cause a group of phenotypically overlapping recessive syndromes with intellectual disability, for which pathogenic mutations have been described in 16 genes of the corresponding molecular pathway. An elevated serum activity of alkaline phosphatase (AP), a GPI-linked enzyme, has been used to assign GPIBDs to the phenotypic series of hyperphosphatasia with mental retardation syndrome (HPMRS) and to distinguish them from another subset of GPIBDs, termed multiple congenital anomalies hypotonia seizures syndrome (MCAHS). However, the increasing number of individuals with a GPIBD shows that hyperphosphatasia is a variable feature that is not ideal for a clinical classification.
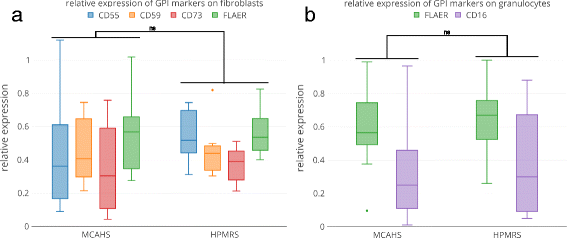
Methods: We studied the discriminatory power of multiple GPI-linked substrates that were assessed by flow cytometry in blood cells and fibroblasts of 39 and 14 individuals with a GPIBD, respectively. On the phenotypic level, we evaluated the frequency of occurrence of clinical symptoms and analyzed the performance of computer-assisted image analysis of the facial gestalt in 91 individuals.
Results: We found that certain malformations such as Morbus Hirschsprung and diaphragmatic defects are more likely to be associated with particular gene defects (PIGV, PGAP3, PIGN). However, especially at the severe end of the clinical spectrum of HPMRS, there is a high phenotypic overlap with MCAHS. Elevation of AP has also been documented in some of the individuals with MCAHS, namely those with PIGA mutations. Although the impairment of GPI-linked substrates is supposed to play the key role in the pathophysiology of GPIBDs, we could not observe gene-specific profiles for flow cytometric markers or a correlation between their cell surface levels and the severity of the phenotype. In contrast, it was facial recognition software that achieved the highest accuracy in predicting the disease-causing gene in a GPIBD.
Conclusions: Due to the overlapping clinical spectrum of both HPMRS and MCAHS in the majority of affected individuals, the elevation of AP and the reduced surface levels of GPI-linked markers in both groups, a common classification as GPIBDs is recommended. The effectiveness of computer-assisted gestalt analysis for the correct gene inference in a GPIBD and probably beyond is remarkable and illustrates how the information contained in human faces is pivotal in the delineation of genetic entities.
Keywords: Anchor biosynthesis defects; Automated image analysis; GPI; Gene; Prediction.
Conflict of interest statement
Consent for publication
Written informed consent was obtained before enrolment from the responsible persons (parents) on behalf of all study participants to publish their photographs as well as their clinical data for scientific purposes.
Competing interests
PMK is a member of the scientific advisory board of FDNA, the company providing the Face2Gene platform. YG and NF are employees of FNDA. The remaining authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures

References
-
- Murakami Y, Kanzawa N, Saito K, Krawitz PM, Mundlos S, Robinson PN, Karadimitris A, Maeda Y, Kinoshita T. Mechanism for release of alkaline phosphatase caused by glycosylphosphatidylinositol deficiency in patients with hyperphosphatasia-mental retardation syndrome. J Biol Chem. 2012;287(9):6318-6325. - PMC - PubMed
-
- Chiyonobu T, Inoue N, Morimoto M, Kinoshita T, Murakami Y. Glycosylphosphatidylinositol (GPI) anchor deficiency caused by mutations in PIGW is associated with West syndrome and hyperphosphatasia with mental retardation syndrome. J Med Genet. 2014;51(3):203–7. doi: 10.1136/jmedgenet-2013-102156. - DOI - PubMed
-
- Hansen L, Tawamie H, Murakami Y, Mang Y, ur Rehman S, Buchert R, Schaffer S, Muhammad S, Bak M, Nothen MM, et al. Hypomorphic mutations in PGAP2, encoding a GPI-anchor-remodeling protein, cause autosomal-recessive intellectual disability. Am J Hum Genet. 2013;92(4):575–83. doi: 10.1016/j.ajhg.2013.03.008. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

