SJS/TEN 2017: Building Multidisciplinary Networks to Drive Science and Translation
- PMID: 29310768
- PMCID: PMC5857362
- DOI: 10.1016/j.jaip.2017.11.023
SJS/TEN 2017: Building Multidisciplinary Networks to Drive Science and Translation
Abstract
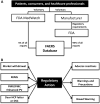
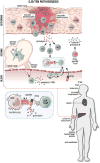
Stevens-Johnson syndrome/toxic epidermal necrolysis (SJS/TEN) is a life-threatening, immunologically mediated, and usually drug-induced disease with a high burden to individuals, their families, and society with an annual incidence of 1 to 5 per 1,000,000. To effect significant reduction in short- and long-term morbidity and mortality, and advance clinical care and research, coordination of multiple medical, surgical, behavioral, and basic scientific disciplines is required. On March 2, 2017, an investigator-driven meeting was held immediately before the American Academy of Dermatology Annual meeting for the central purpose of assembling, for the first time in the United States, clinicians and scientists from multiple disciplines involved in SJS/TEN clinical care and basic science research. As a product of this meeting, this article summarizes the current state of knowledge and expert opinion related to SJS/TEN covering a broad spectrum of topics including epidemiology and pharmacogenomic networks; clinical management and complications; special populations such as pediatrics, the elderly, and pregnant women; regulatory issues and the electronic health record; new agents that cause SJS/TEN; pharmacogenomics and immunopathogenesis; and the patient perspective. Goals include the maintenance of a durable and productive multidisciplinary network that will significantly further scientific progress and translation into prevention, early diagnosis, and management of SJS/TEN.
Keywords: Electronic health record; Granulysin; HLA; Networks; Pharmacogenomics; Pharmacovigilance; Stevens-Johnson; T cells; Toxic epidermal necrolysis.
Copyright © 2017 American Academy of Allergy, Asthma & Immunology. All rights reserved.
Figures











Comment in
-
Identifying SJS/TEN using ICD-9-CM coding - Real or just fantasy?Allergol Int. 2020 Jan;69(1):136-137. doi: 10.1016/j.alit.2019.06.006. Epub 2019 Jul 6. Allergol Int. 2020. PMID: 31288967 No abstract available.
References
-
- Manolio TA, Hutter CM, Avigan M, Cibotti R, Davis RL, Denny JC, Grenade LL, Wheatley LM, Carrington MN, Wasun C, Chung WH, Dalton AD, Hung SI, Lee MTM, Leeder JS, Lertora JJL, Mahasirimongkol S, McLeod HL, Mockenhaupt M, Pacanowski M, Phillips EJ, Pinheiro S, Pirmohamed M, Sung C, Suwankesawong W, Trepanier L, Tumminia SJ, Veenstra D, Yuliwulandari R, Shear NH. Research directions in genetic predispositions to Stevens-Johnson syndrome/toxic epidermal necrolysis. Clinical Pharmacology and Therapeutics. 2017 (in press) - PMC - PubMed
-
- Maverakis E, Wang EA, Shinkai K, Mahasirimongkol S, Margolis DJ, Avigan M, et al. Stevens-Johnson Syndrome and Toxic Epidermal Necrolysis Standard Reporting and Evaluation Guidelines: Results of a National Institutes of Health Working Group. JAMA dermatology. 2017 Jun 01;153(6):587–92. - PubMed
-
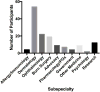
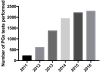
- Ergen ENBJ, Holmes JH, Ye F, Luo L, Phillips EJ. Foundations of a North American SJS/TEN Research Network: results of a web-based survey of dermatologists and surgeons. American Burn Association (ABA) 49th Annual Meeting; Boston, MA. 2017.
-
- Bastuji-Garin S, Fouchard N, Bertocchi M, Roujeau JC, Revuz J, Wolkenstein P. SCORTEN: a severity-of-illness score for toxic epidermal necrolysis. The Journal of investigative dermatology. 2000 Aug;115(2):149–53. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

