Matrix Metalloproteinases, Vascular Remodeling, and Vascular Disease
- PMID: 29310800
- PMCID: PMC5765875
- DOI: 10.1016/bs.apha.2017.08.002
Matrix Metalloproteinases, Vascular Remodeling, and Vascular Disease
Abstract
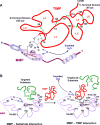
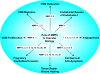
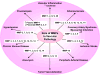
Matrix metalloproteinases (MMPs) are a family of zinc-dependent endopeptidases that degrade various proteins in the extracellular matrix (ECM). Typically, MMPs have a propeptide sequence, a catalytic metalloproteinase domain with catalytic zinc, a hinge region or linker peptide, and a hemopexin domain. MMPs are commonly classified on the basis of their substrates and the organization of their structural domains into collagenases, gelatinases, stromelysins, matrilysins, membrane-type (MT)-MMPs, and other MMPs. MMPs are secreted by many cells including fibroblasts, vascular smooth muscle (VSM), and leukocytes. MMPs are regulated at the level of mRNA expression and by activation through removal of the propeptide domain from their latent zymogen form. MMPs are often secreted in an inactive proMMP form, which is cleaved to the active form by various proteinases including other MMPs. MMPs degrade various protein substrates in ECM including collagen and elastin. MMPs could also influence endothelial cell function as well as VSM cell migration, proliferation, Ca2+ signaling, and contraction. MMPs play a role in vascular tissue remodeling during various biological processes such as angiogenesis, embryogenesis, morphogenesis, and wound repair. Alterations in specific MMPs could influence arterial remodeling and lead to various pathological disorders such as hypertension, preeclampsia, atherosclerosis, aneurysm formation, as well as excessive venous dilation and lower extremity venous disease. MMPs are often regulated by endogenous tissue inhibitors of metalloproteinases (TIMPs), and the MMP/TIMP ratio often determines the extent of ECM protein degradation and tissue remodeling. MMPs may serve as biomarkers and potential therapeutic targets for certain vascular disorders.
Keywords: Aneurysm; Angiogenesis; Atherosclerosis; Cell signaling; Extracellular matrix; Hypertension; Smooth muscle; TIMPs.
© 2018 Elsevier Inc. All rights reserved.
Conflict of interest statement
None
Figures


References
-
- Abdalvand A, Morton JS, Bourque SL, Quon AL, Davidge ST. Matrix metalloproteinase enhances big-endothelin-1 constriction in mesenteric vessels of pregnant rats with reduced uterine blood flow. Hypertension. 2013;61:488–493. - PubMed
-
- Aguilera CM, George SJ, Johnson JL, Newby AC. Relationship between type IV collagen degradation, metalloproteinase activity and smooth muscle cell migration and proliferation in cultured human saphenous vein. Cardiovasc Res. 2003;58:679–688. - PubMed
-
- Ahn HS, Chackalamannil S, Boykow G, Graziano MP, Foster C. Development of proteinase-activated receptor 1 antagonists as therapeutic agents for thrombosis, restenosis and inflammatory diseases. Curr Pharm Des. 2003;9:2349–2365. - PubMed
-
- Aikawa M, Rabkin E, Voglic SJ, Shing H, Nagai R, Schoen FJ, Libby P. Lipid lowering promotes accumulation of mature smooth muscle cells expressing smooth muscle myosin heavy chain isoforms in rabbit atheroma. Circ Res. 1998;83:1015–1026. - PubMed
-
- Aimes RT, Quigley JP. Matrix metalloproteinase-2 is an interstitial collagenase. Inhibitor-free enzyme catalyzes the cleavage of collagen fibrils and soluble native type I collagen generating the specific 3/4- and 1/4-length fragments. J Biol Chem. 1995;270:5872–5876. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

