Novel Pyrimidines as Antitubercular Agents
- PMID: 29311070
- PMCID: PMC5826157
- DOI: 10.1128/AAC.02063-17
Novel Pyrimidines as Antitubercular Agents
Abstract
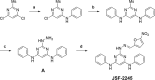
Mycobacterium tuberculosis infection is responsible for a global pandemic. New drugs are needed that do not show cross-resistance with the existing front-line therapeutics. A triazine antitubercular hit led to the design of a related pyrimidine family. The synthesis of a focused series of these analogs facilitated exploration of their in vitro activity, in vitro cytotoxicity, and physiochemical and absorption-distribution-metabolism-excretion properties. Select pyrimidines were then evaluated for their pharmacokinetic profiles in mice. The findings suggest a rationale for the further evolution of this promising series of antitubercular small molecules, which appear to share some similarities with the clinical compound PA-824 in terms of activation, while highlighting more general guidelines for the optimization of small-molecule antitubercular agents.
Keywords: Mycobacterium tuberculosis; antitubercular; pharmacokinetics; pyrimidine.
Copyright © 2018 American Society for Microbiology.
Figures
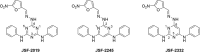


References
-
- World Health Organization. 2016. Global tuberculosis report. WHO, Geneva, Switzerland: http://www.who.int/tb/publications/global_report/en/.
-
- Ekins S, Reynolds RC, Kim H, Koo M-S, Ekonomidis M, Talaue M, Paget SD, Woolhiser LK, Lenaerts AJ, Bunin BA, Connell N, Freundlich JS. 2013. Bayesian models leveraging bioactivity and cytotoxicity information for drug discovery. Chem Biol 20:370–378. doi:10.1016/j.chembiol.2013.01.011. - DOI - PMC - PubMed
-
- Baiazitov R, Du W, Lee C-S, Hwang S, Almstead NG, Moon Y-C. 2013. Chemoselective reactions of 4,6-dichloro-2-(methylsulfonyl)pyrimidine and related electrophiles with amines. Synthesis 45:1764–1784. doi:10.1055/s-0033-1338853. - DOI
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

