Crystal Structure of NisI in a Lipid-Free Form, the Nisin Immunity Protein, from Lactococcus lactis
- PMID: 29311076
- PMCID: PMC5826101
- DOI: 10.1128/AAC.01966-17
Crystal Structure of NisI in a Lipid-Free Form, the Nisin Immunity Protein, from Lactococcus lactis
Abstract
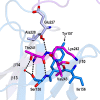
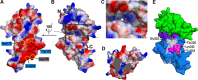
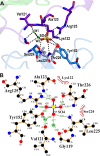
Nisin is a lantibiotic, a member of a family of polypeptides containing lanthionine with antimicrobial activity. Nisin-producing microorganisms require immunity proteins for self-protection from nisin itself. Lactococcus lactis, a microorganism that synthesizes nisin, has an integral NisFEG ABC transporter and an NisI lipoprotein that function in nisin immunity. Here, we present the crystal structure of the full length of NisI22-C, a lipid-free form of NisI, determined at 1.9-Å resolution. As with the nuclear magnetic resonance (NMR) structures of the N- and C-terminal domains of NisI, NisI22-C is composed of N- and C-terminal domains, both of which display a fold similar to that found in SpaI, a lipoprotein with immunity against subtilin in Bacillus subtilis The full-length structure of NisI22-c reveals a large, deep cleft by the interdomain association, one side of which is occupied by the residues important for immunity. Opposite the cleft, a shallow groove is found where nisin-interacting residues are distributed in the periphery composed of the C-terminal negative patch. Based on a sulfate ion found in the large and deep cleft, a model of NisI in complex with a farnesyl diphosphate backbone of lipid II is proposed, suggesting a mechanism for increasing the chances of encountering nisin.
Keywords: Lactococcus lactis; NisI; SpaI; X-ray crystallography; crystal structure; immunity protein; lantibiotics; nisin.
Copyright © 2018 American Society for Microbiology.
Figures

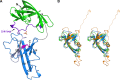

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

