Cessation from Smoking Improves Innate Host Defense and Clearance of Experimentally Inoculated Nasal Staphylococcus aureus
- PMID: 29311241
- PMCID: PMC5865040
- DOI: 10.1128/IAI.00912-17
Cessation from Smoking Improves Innate Host Defense and Clearance of Experimentally Inoculated Nasal Staphylococcus aureus
Abstract
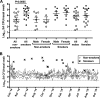
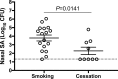
Staphylococcus aureus nasal carriage is transient in most humans and usually benign, but dissemination of S. aureus to extranasal sites causes the majority of clinical infections, and S. aureus is a major cause of serious infections in the United States. A better understanding of innate nasal decolonization mechanisms is urgently needed, as are relevant models for studying S. aureus clearance. Here, we screened a population of healthy smokers for nasal S. aureus carriage and compared the participants' abilities to clear experimentally applied nasal S. aureus before and after completion of a smoking cessation program. We determined that cigarette smoking increases the mean nasal S. aureus load (2.6 × 104 CFU/swab) compared to the load observed in healthy nonsmokers (1.7 × 103 CFU/swab) and might increase the rate of S. aureus nasal carriage in otherwise-healthy adults: 22 of 99 smokers carried S. aureus at the screening visit, while only 4 of 30 nonsmokers screened positive during the same time period. Only 6 of 19 experimental inoculation studies in active smokers resulted in S. aureus clearance within the month of follow-up, while in the cessation group, 6 of 9 subjects cleared nasal S. aureus and carriage duration averaged 21 ± 4 days. Smoking cessation associated with enhanced expression of S. aureus-associated interleukin-1β (IL-1β) and granulocyte colony-stimulating factor (G-CSF) in nasal fluids. Participants who failed to clear S. aureus exhibited a higher nasal S. aureus load and elevated nasal interleukin-1 receptor antagonist (IL-1RA) expression at the preexperiment study visits. We conclude that smokers exhibit higher S. aureus loads than nonsmokers and that innate immune pathways, including G-CSF expression and signaling through the IL-1 axis, are important mediators of nasal S. aureus clearance.
Keywords: Staphylococcus aureus; experimental colonization; host defense; humans; nasal carriage; smoking cessation.
Copyright © 2018 American Society for Microbiology.
Figures




References
-
- Miller RR, Walker AS, Godwin H, Fung R, Votintseva A, Bowden R, Mant D, Peto TE, Crook DW, Knox K. 2014. Dynamics of acquisition and loss of carriage of Staphylococcus aureus strains in the community: the effect of clonal complex. J Infect 68:426–439. doi: 10.1016/j.jinf.2013.12.013. - DOI - PMC - PubMed
-
- Kock R, Werner P, Friedrich AW, Fegeler C, Becker K, Prevalence of Multiresistant Microorganisms Study Group. 2016. Persistence of nasal colonization with human pathogenic bacteria and associated antimicrobial resistance in the German general population. New Microbes New Infect 9:24–34. doi: 10.1016/j.nmni.2015.11.004. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

