mTOR signaling in stem and progenitor cells
- PMID: 29311260
- PMCID: PMC5825873
- DOI: 10.1242/dev.152595
mTOR signaling in stem and progenitor cells
Abstract
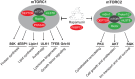
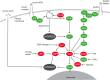
The mammalian target of rapamycin (mTOR) senses nutrients and growth factors to coordinate cell growth, metabolism and autophagy. Extensive research has mapped the signaling pathways regulated by mTOR that are involved in human diseases, such as cancer, and in diabetes and ageing. Recently, however, new studies have demonstrated important roles for mTOR in promoting the differentiation of adult stem cells, driving the growth and proliferation of stem and progenitor cells, and dictating the differentiation program of multipotent stem cell populations. Here, we review these advances, providing an overview of mTOR signaling and its role in murine and human stem and progenitor cells.
Keywords: Amino acids; Metabolism; Signaling; Stem cell; mTOR.
© 2018. Published by The Company of Biologists Ltd.
Conflict of interest statement
Competing interestsThe authors declare no competing or financial interests.
Figures


References
-
- Bar-Peled L., Chantranupong L., Cherniack A. D., Chen W. W., Ottina K. A., Grabiner B. C., Spear E. D., Carter S. L., Meyerson M. and Sabatini D. M. (2013). A Tumor suppressor complex with GAP activity for the Rag GTPases that signal amino acid sufficiency to mTORC1. Science 340, 1100-1106. 10.1126/science.1232044 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

